"Determination of the electron charge. Determination of elemental electric charge by electrolysis Measurement of elemental charge
Methodical note... Students already know about the electron from the chemistry course and the corresponding section of the VII grade program. Now it is necessary to deepen the understanding of the first elementary particle of matter, recall what has been studied, connect it with the first topic of the section "Electrostatics" and move on to a higher level of interpretation of the elementary charge. It should be borne in mind the complexity of the concept of electric charge. The proposed excursion can help reveal this concept and get to the heart of the matter.
Electron has a complex history. To arrive at the goal in the shortest possible way, it is advisable to lead the story as follows.
The discovery of the electron was the result of numerous experiments. By the beginning of the XX century. the existence of the electron has been established in a number of independent experiments. But, in spite of the colossal experimental material accumulated by entire national schools, the electron remained a hypothetical particle, for experience had not yet answered a number of fundamental questions.
First of all, there was not a single experiment in which individual electrons would participate. The elementary charge was calculated on the basis of measurements of the microscopic charge, assuming the validity of a number of hypotheses.
Uncertainty was at a crucial point. First, the electron appeared as a result of the atomistic interpretation of the laws of electrolysis, then it was discovered in a gas discharge. It was not clear whether physics was actually dealing with the same object. A large group of skeptical natural scientists believed that the elementary charge is the statistical average of charges of the most diverse magnitudes. Moreover, none of the experiments on measuring the electron charge gave strictly repeated values.
There were skeptics who generally ignored the discovery of the electron. Academician AF Ioffe, in his memoirs about his teacher VK Roentgen, wrote: “Until 1906-1907 the word electron should not have been pronounced at the Physics Institute of the University of Munich. needs".
The question of the mass of the electron has not been resolved; it has not been proven that charges on both conductors and dielectrics consist of electrons. The concept of "electron" did not have an unambiguous interpretation, for the experiment had not yet revealed the structure of the atom (Rutherford's planetary model would appear in 1911, and Bohr's theory in 1913).
The electron has not yet entered into theoretical constructions. The electron theory of Lorentz featured a continuously distributed charge density. In the theory of metallic conductivity, developed by Drude, it was about discrete charges, but these were arbitrary charges, on the value of which no restrictions were imposed.
The electron has not yet gone beyond the framework of "pure" science. Recall that the first electronic tube appeared only in 1907.
For the transition from faith to conviction, it was necessary first of all to isolate the electron, to invent a method for direct and accurate measurement of the elementary charge.
This problem was solved by the American physicist Robert Millikan (1868-1953) in a series of subtle experiments that began in 1906.
Robert Millikan was born in 1868 in Illinois to a poor priestly family. He spent his childhood in the provincial town of Macvocket, where much attention was paid to sports and poorly taught. A high school principal who taught physics, said, for example, to his young students: "How can you make sound out of waves? Nonsense, boys, all this is nonsense!"
Oberdeen College was no better, but Millikan, who had no financial support, had to teach physics in high school himself. In America then there were only two textbooks on physics, translated from French, and the talented young man did not have any difficulties to study them and successfully teach. In 1893 he entered Columbia University, then went to study in Germany.
Millikan was 28 years old when he received an offer from A. Michelson to take an assistant position at the University of Chicago. At the beginning, he was engaged here almost exclusively in pedagogical work and only at the age of forty began scientific research, which brought him worldwide fame.
The first experiments boiled down to the following. Between the plates of the flat capacitor, to which a voltage of 4000 V was applied, a cloud was created, consisting of water droplets deposited on the ions. The top of the cloud was first observed to fall in the absence of an electric field. Then a cloud was created with the voltage turned on. The fall of the cloud took place under the influence of gravity and electrical force.
The ratio of the force acting on a drop in a cloud to the speed it acquires is the same in the first and second cases. In the first case, the force is mg, in the second mg + qE, where q is the drop's charge, E is the electric field strength. If the speed in the first case is equal to v 1 in the second v 2, then
Knowing the dependence of the speed of falling of the cloud v on the viscosity of the air, we can calculate the required charge q. However, this method did not give the desired accuracy, because it contained hypothetical assumptions that were beyond the control of the experimenter.
In order to increase the accuracy of measurements, it was first of all necessary to find a way to take into account the evaporation of the cloud, which inevitably occurred during the measurement.
Reflecting on this problem, Millikan came up with the classical drop method, which opened up a number of unexpected possibilities. We will leave the story of the invention to the author himself:
“Realizing that the rate of evaporation of the droplets remained unknown, I tried to come up with a method that would completely eliminate this indeterminate value. My plan was as follows. In previous experiments, the electric field could only slightly increase or decrease the rate of falling of the top of the cloud under the influence of gravity. Now I wanted to amplify that field so that the upper surface of the cloud remained at a constant height. In this case, it became possible to accurately determine the rate of evaporation of the cloud and take it into account in the calculations. " To implement this idea, Millikan designed a small-sized rechargeable battery, which gave a voltage of up to 104 V (for that time this was an outstanding achievement of the experimenter). She had to create a field strong enough for the cloud to be held, like the "coffin of Mohammed", in limbo.
“When I had everything ready,” says Millikan, “and when the cloud formed, I turned on the switch, and the cloud was in the electric field. , which could be observed with a control optical device, as Wilson did and I was going to do. As it seemed to me at first, the disappearance of the cloud without a trace in the electric field between the upper and lower plates meant that the experiment ended in vain ... "
However, as has often happened in the history of science, failure gave birth to a new idea. She led to the famous drop method. "Repeated experiments," writes Millikan, "showed that after the cloud was scattered in a powerful electric field, in its place it was possible to distinguish several separate water drops" (emphasized by me - V.D.).
The "unsuccessful" experiment led to the discovery of the possibility of keeping in equilibrium and observing individual droplets for a sufficiently long time.
But during the observation period, the mass of the water droplet changed significantly as a result of evaporation, and Millikan, after many days of searching, switched to experiments with oil droplets.
The experimental procedure turned out to be simple. A cloud is formed between the condenser plates by adiabatic expansion. It consists of droplets with charges of different magnitude and sign. When the electric field is turned on, drops with charges of the same name as the charge on the upper plate of the capacitor quickly fall, and drops with the opposite charge are attracted by the upper plate. But a certain number of drops have such a charge that the force of gravity is balanced by the electrical force.
After 7 or 8 minutes, the cloud dissipates, and a small number of drops remain in the field of view, the charge of which corresponds to the said balance of forces.
Millikan observed these drops as distinct bright points. “The history of these drops usually proceeds like this,” he writes. “In the case of a slight predominance of gravity over the force of the field, they begin to slowly fall, but since they gradually evaporate, their downward movement soon ceases, and they become motionless for quite a long time. Then the field begins to prevail, and the drops begin to rise slowly. Towards the end of their life in the space between the plates, this upward movement becomes very strongly accelerated, and they are attracted at high speed to the upper plate. "
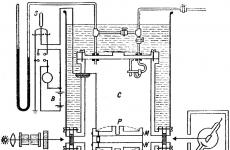
The diagram of the Millikan apparatus, with the help of which decisive results were obtained in 1909, is shown in Figure 17.

Chamber C contained a flat capacitor made of round brass plates M and N with a diameter of 22 cm (the distance between them was 1.6 cm). A small hole p was made in the center of the top plate through which the oil droplets passed. The latter were formed by blowing in a jet of oil using a spray. In this case, the air was preliminarily purified from dust by passing it through a pipe with glass wool. The oil droplets had a diameter of about 10-4 cm.
A voltage of 104 V was supplied from the storage battery B to the capacitor plates. Using a switch, it was possible to short-circuit the plates and thereby destroy the electric field.
Oil drops falling between plates M and N were illuminated by a strong source. The behavior of the drops was observed perpendicular to the direction of the rays through the telescope.
The ions necessary for the condensation of the droplets were created by the radiation of a piece of radium 200 mg in mass located at a distance of 3 to 10 cm from the side of the plates.
With the help of a special device, the gas was expanded by lowering the piston. After 1–2 s after expansion, the radium was removed or obscured by a lead shield. Then the electric field was turned on and the observation of drops into the telescope began.
The pipe had a scale by which it was possible to count the distance traveled by the drop for a certain period of time. The time was recorded by an accurate watch with a lock.
In the course of observations, Millikan discovered a phenomenon that served as the key to the entire series of subsequent accurate measurements of individual elementary charges.
“While working on suspended droplets,” writes Millikan, “I forgot to block them from the radium rays several times. in the first case it was a positive and in the second case it was a negative ion.
Indeed, measuring the velocity of the same drop twice, once before and the second time after the capture of the ion, I could obviously completely exclude the properties of the drop and the properties of the medium and operate with a quantity proportional only to the charge of the captured ion. "
The elementary charge was calculated by Millikan based on the following considerations. The speed of the drop is proportional to the force acting on it and does not depend on the charge of the drop.
If a drop fell between the plates of the capacitor under the action of only gravity with a velocity v 1, then
When the field is turned on, directed against the force of gravity, the acting force will be the difference qE = mg, where q is the charge of the drop, E is the modulus of the field strength.
The droplet speed will be equal to:
v 2 = k (qE - mg) (2)
If we divide equality (1) by (2), we get


Let the drop capture an ion and its charge becomes equal to q 'and the speed of motion v 2'. The charge of this trapped ion is denoted by e. Then e = q ′ - q.
Using (3), we obtain

The value is constant for a given drop.
Consequently, any charge captured by the drop will be proportional to the difference in velocities (v ′ 2 -v 2), in other words, proportional to the change in the droplet velocity due to the capture of an ion!
So, the measurement of the elementary charge was reduced to the measurement of the distance traveled by the drop and the time during which this distance was covered.
Numerous observations have shown the validity of formula (4). It turned out that the value of e can only change in jumps! Charges e, 2e, 3e, 4e, etc. are always observed.
“In many cases,” writes Millikan, “the drop was observed for five or six hours, and during this time it captured not eight or ten ions, but hundreds of them. In total, I observed the capture of many thousands of ions in this way, and in all cases, the captured charge ... was either exactly equal to the smallest of all captured charges, or it was equal to a small integer multiple of this value.This is direct and irrefutable proof that the electron is not a "statistical average", but that all electric charges on ions are either exactly equal to the charge of an electron, or are small integer multiples of that charge. "
So, atomism, discreteness, or, in modern terms, the quantization of an electric charge has become an experimental fact. Now it was important to show that the electron is, so to speak, omnipresent. Any electric charge in a body of any nature is the sum of the same elementary charges.
Millikan's method made it possible to unequivocally answer this question.
In the first experiments, charges were created by ionizing neutral gas molecules with a stream of radioactive radiation. The charge of ions captured by the droplets was measured.
When liquid is sprayed with a spray bottle, the droplets are electrified due to friction. This was well known back in the 19th century. Are these charges as quantized as the charges of the ions?
Millikan "weighs" the droplets after spraying and measures the charges in the manner described above. Experience reveals the same discreteness of the electric charge.
By spraying drops of oil (dielectric), glycerin (semiconductor), mercury (conductor), Millikan proves that charges on bodies of any physical nature consist in all cases without exception of individual elementary portions of a strictly constant value.
In 1913 Millikan summarized the results of numerous experiments and gave the following value for an elementary charge: e = 4.774 · 10 -10 units. CGSE charge.
This is how one of the most important constants of modern physics was established. Determining the electric charge has become a simple arithmetic problem.
Electron visualization... An important role in strengthening the idea of the reality of the electron was played by the discovery by G.A. Wilson of the effect of condensation of water vapor on ions, which led to the possibility of photographing particle tracks.
They say that A. Compton at the lecture could in no way convince a skeptical listener of the reality of the existence of microparticles. He insisted that he would believe only when he saw them with his own eyes.
Then Compton showed the photograph with an alpha-particle track, next to which was a fingerprint. "Do you know what this is?" Compton asked. “Finger,” the listener replied. "In that case," Compton declared solemnly, "this strip of light is the particle."
The photographs of the tracks of the electrons were not only indicative of the reality of the electrons. They confirmed the assumption about the small size of the electrons and made it possible to compare with experiment the results of theoretical calculations, in which the electron radius appeared. The experiments, which were initiated by Lenard in the study of the penetrating power of cathode rays, showed that very fast electrons emitted by radioactive substances give tracks in the gas in the form of straight lines. The length of the track is proportional to the energy of the electron. The photographs of the high energy alpha particle tracks show that the tracks are composed of a large number of dots. Each point is a water droplet appearing on an ion, which is formed as a result of the collision of an electron with an atom. Knowing the size of the atom and their concentration, we can calculate the number of atoms through which the α-particle must pass at a given distance. A simple calculation shows that an α-particle must pass about 300 atoms before it meets one of the electrons that make up the atomic shell on its way and ionizes.
This fact convincingly indicates that the volume of electrons is a negligible fraction of the volume of an atom. The track of a low-energy electron is curved; therefore, a slow electron is deflected by the intra-atomic field. It produces more ionization acts on its way.
From scattering theory, data can be obtained to estimate the deflection angles as a function of the electron energy. These data are well supported by the analysis of real tracks. The coincidence of theory with experiment has strengthened the idea of the electron as the smallest particle of matter.
Measurement of the elementary electric charge opened up the possibility of precise determination of a number of the most important physical constants.
Knowing the value of e automatically makes it possible to determine the value of the fundamental constant - Avogadro's constant. Before Millikan's experiments, there were only rough estimates of the Avogadro constant, which were given by the kinetic theory of gases. These estimates were based on calculations of the average radius of an air molecule and varied within a fairly wide range from 2 · 10 23 to 20 · 10 23 1 / mol.
Let us assume that we know the charge Q that has passed through the electrolyte solution and the amount of substance M that is deposited on the electrode. Then, if the charge of the ion is Ze 0 and its mass is m 0, the equality

If the mass of the deposited substance is equal to one mole, then Q = F is the Faraday constant, and F = N 0 e, whence N 0 = F / e. Obviously, the accuracy of determining the Avogadro constant is given by the accuracy with which the electron charge is measured.
Practice required an increase in the accuracy of determining the fundamental constants, and this was one of the incentives to continue improving the method for measuring the quantum of electric charge. This work, which is already of a purely metrological nature, continues to this day.
The most accurate values are currently:
e = (4.8029 ± 0.0005) 10 -10 units. CGSE charge;
N 0 = (6.0230 ± 0.0005) 10 23 1 / mol.
Knowing N 0, it is possible to determine the number of gas molecules in 1 cm 3, since the volume occupied by 1 mole of gas is an already known constant.
Knowing the number of gas molecules in 1 cm 3, in turn, made it possible to determine the average kinetic energy of the thermal motion of a molecule.
Finally, the electron charge can be used to determine the Planck constant and the Stefan-Boltzmann constant in the law of thermal radiation.
The work was added to the site site: 2016-03-13Is free
Find out the cost of work
Laboratory robot
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ELEMENTARY CHARGE AND MILLIKEN'S EXPERIENCE
; font-family: "Arial" "xml: lang =" uk-UA "lang =" uk-UA "> Robot target; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">:; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> study of the motion of charged drops in electric and gravitational fields (Millikan's experiment). Determination of the elementary charge.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Equipment; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">: Millikan device, multimeter, voltage source 0 ÷ 600 V, micrometer 1 mm - 100 divisions, 2 stopwatches, glasses 18 x 18 mm, switch, tripod, tube.
; font-family: "Arial"; text-decoration: underline "xml: lang =" ru-RU "lang =" ru-RU "> Determination of the radii and charges of charged drops. Measurement of the speed of movement of drops at various voltages and directions of the electric field ...
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 1. Switch on the optical system of the Millikan installation and calibrate the micrometer using a special graduation glass.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 2. Set the voltage to 300 V on the Millikan installation. Inject oil droplets into the observation space in the installation. Adjusting the optical system slightly, observe the movement of the oil droplets. To change the direction of the droplets, use the switch to change the direction of the electric field. From the visible droplets, select the one that moves strictly vertically and at a low speed. Since the sizes of the resulting droplets are small, it can be considered with a high degree of accuracy that the observed movement is already steady (the droplet moves at a constant speed).
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 3. Determine the movement time using the stopwatch; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> t; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> the selected drop up when passing a certain distance; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> S; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, as well as the movement time; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> t; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> the same drop down when passing a certain distance; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> S; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">. The distance traveled by a drop is determined as the product of the micrometer division value (see item 1 of the task) by the number of scale divisions passed. Enter the data in Table 1. Repeat the experiment with a few drops (4 ÷ 6 drops).
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Table 1.
|
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> No drop |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> U; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, В |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> S; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 1; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> mm |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> t; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 1; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> с |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> S; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 2; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> mm |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> t; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, with |
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 4. Repeat the experiment for a few drops (4 ÷ 6 drops) at voltages on the Milliken apparatus of 400 V and 500 V. Fill in the data in table 1.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 5. Using the data in Table 1, calculate the speeds; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> drops according to formulas (6) and (7) and, then, radii and charges of drops according to formulas (8) and (9). Since the charge of a drop is an integer; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> n; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> elementary charge; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> (electron charge):
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> (; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">)
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> then you can determine this elementary charge. Fill in table 2.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Table 2.
|
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> No drop |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, m / s |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, m / s |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> Q; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, cl |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> r; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> м |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> n |
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, Кл |
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 6. Perform mathematical processing of the results. Hold the graph. An example of the experiment is shown in Fig. 1.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 7. Analyze the results obtained and formulate conclusions in accordance with the guidelines. Pay attention to the consistency of the conclusions with the set goal.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Fig. 1.; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> An example of an experiment to determine the charge of various drops; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">
Brief theoretical materials
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> The idea of the discreteness of the electric charge was first expressed by B. Franklin (1752). The discreteness of the charges was experimentally substantiated by M. Faraday (1834) based on the laws of electrolysis The numerical value of the elementary charge (the smallest electric charge found in nature) was theoretically calculated using Avogadro's number A direct experimental measurement of the elementary charge was carried out by R. Millikan (1908-1916) using method of oil droplets The method is based on the study of the motion of charged oil droplets in a uniform electric field of known strength; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Ē; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">. According to the basic concepts of electronic theory, the charge of a body changes as a result of a change in the number of electrons contained in it (or, in some phenomena, ions, the magnitude of the charge of which is a multiple of the charge of an electron.) Therefore, the charge of any body must change abruptly and in portions that contain an integer number of electron charges.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Millikan measured the electric charge concentrated on individual small spherical droplets that were formed by the spray; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> P; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and acquired an electric charge by electrification due to friction against the walls of the atomizer, as shown in Fig. 2. Through a small hole in the upper plate flat capacitor; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> K; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> they fell into the space between the plates. The movement of the drop was observed through a microscope; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> M; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Fig. 2:; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Installation diagram. P - droplet atomizer, K - condenser, IP - power supply, M - microscope, h; font-family: "Symbol" "xml: lang =" ru-RU "lang =" ru-RU "> ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - radiation source, P - table surface.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">
In order to protect droplets from convection air currents, the condenser was enclosed in a protective casing, the temperature and pressure in which were kept constant. When performing the experiments, it was required to comply with the following conditions:
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 1. Drops must be microscopic in order to:
- ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> the electrostatic force acting on the charged drop, when the electric field was on, exceeded the force of gravity;
- ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> the drop charge, as well as its changes during irradiation (using an ionizer) were equal to a fairly small number of elementary charges.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> This makes it easier to set the multiplicity of the drop charge to an elementary charge;
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 2. Drop density; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> = 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, 03 * 10; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> kg / m; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> must be greater than the density of a viscous medium; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, in which it moves (air -; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> = 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, 293 kg / m; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">);
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 3. The mass of the drop should not change during the entire experiment. For this, the oil that makes up the drop should not evaporate (oil evaporates much more slowly than water).
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> If the capacitor plates were not charged (electric field strength; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Ē; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> = 0), then the drop slowly fell, moving from the top plate to the bottom.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> As soon as the capacitor plates were charged, there were changes in the movement of the drop: in the case of a negative charge on the drop and a positive charge on the top plate of the capacitor the drop slowed down, and at some point in time it changed its direction of motion to the opposite - it began to rise to the upper plate.
The equation of motion for a drop
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Knowing the drop rate in the absence of an electrostatic field (its charge did not play a role) and the drop rate in a given and known electrostatic field, Millikan could calculate the charge of the drop.To determine the charge, it is necessary to consider first the movement of the drop in the absence of an electrostatic field; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Ē; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> = 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">) The balance of power is shown in Figure 3.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> In this case, three forces act on the drop (see Fig. 3.a):
- ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> gravity; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> mg, g; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 9.81 m / s; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">;
- ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Archimedean force; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Vg; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> m; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> g; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> F; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> A; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">,
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> where; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - air density,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> V; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> = (4/3); font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> πr; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - drop volume,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> V; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> m; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - the mass of air displaced by the drop;
- ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> viscous resistance force expressed by Stokes' formula; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> kv; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 6; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> πηrv; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> FC; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, where; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> η; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> = 1.82 * 10; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> - 5; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> kg / m * s - air viscosity,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> r; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - drop radius,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - drop speed.
; font-family: "Arial"; text-decoration: underline "xml: lang =" ru-RU "lang =" ru-RU "> Note; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">: The Stokes formula is valid for a ball moving in gas, provided that the radius of the ball is many times greater than the free path In Millikan's experiment, the droplets were so small that he had to introduce the necessary corrections into the calculations. the effective density of a droplet can differ significantly from the density of its substance.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 2 Newton's law projected onto the axis; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> X; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> for the case corresponding to Fig. 3.a:
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 0; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">); font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> g; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> +; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> kv; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> g; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> =; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> - ma; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (2)
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> where; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> a; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - the acceleration with which the drop falls.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Due to viscous resistance, the drop almost immediately after the start of movement or a change in the conditions of movement acquires a constant (steady) speed and moves uniformly . Because of this; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> a; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> = 0, and from (1) you can find the speed of the droplet. Let's denote the modulus of the steady-state speed in the absence of an electrostatic field; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> g; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">. Then:
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> g; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> = (; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 0; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">); font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> g; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> /; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> k; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (3)
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> If you close the electrical circuit of a capacitor (Fig. 3.b), then it will be charged and an electrostatic field will be created in it; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Ē; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">.; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> q; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> (let it be positive) additional force will act; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> qE; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, directed upwards (Fig. 3.b).
- "xml: lang =" uk-UA "lang =" uk-UA "> force from the side of the electric field (the field of a charged capacitor), where is the charge of the drop,Ē - electric field strength, U - voltage across the capacitor plates, d is the distance between the plates.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> a); font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> b); font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Fig. 3:; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Forces acting on the drop:; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> a); font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> in the absence of an electrostatic field;; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> b); font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> in the presence of an electrostatic field.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> As in the case of free fall of a drop, consider the steady state of motion. Newton's law in projection onto the axis; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> X; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and taking into account that; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> a; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> = 0, will take the form:
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 0; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">); font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> g; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> +; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> qE; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> +; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> kv; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> = 0 (4)
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> = [; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> - q; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> m; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 0; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">); font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> g; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">]; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> /; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> k; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (5)
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> where; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> E; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - steady-state speed of an oil drop in the electrostatic field of a capacitor:; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">< ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 0, if the drop moves down,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">>; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 0 if the blob moves up.
"xml: lang =" ru-RU "lang =" ru-RU "> (6)
"xml: lang =" ru-RU "lang =" ru-RU "> (7)
; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> From formulas (6) and (7) you can get formulas for determining the charge and radius of drops through the speed of the droplet up and down:
; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU ">,; font-family: "Arial"; color: # 000000 "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> (8)
; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> where kg m; font-family: "Arial"; vertical-align: super; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> 0.5; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> с; font-family: "Arial"; vertical-align: super; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> - 0.5; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> and
; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU ">,; font-family: "Arial"; color: # 000000 "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> (9)
; font-family: "Arial"; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> where (ms); font-family: "Arial"; vertical-align: super; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> 0.5
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Determination of the elementary charge by means of a computational experiment
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> From equation (5) it follows that by measuring steady-state velocities; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> g; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> E; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> in the absence of an electrostatic field and in its presence, respectively, it is possible to determine the drop charge if the coefficient is known; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> k; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 6; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> πηr; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">. It would seem to find; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> k; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> it is enough to measure the radius of the drop (the viscosity of air is known from other experiments). However, direct measurement of this radius using a microscope is impossible:; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> r; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> has an order of magnitude of 10; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 4; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ÷; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 10; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 6; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> cm, which is comparable to the light wavelength. Therefore, the microscope gives only a diffraction image of the drop, not allowing to measure its actual size. Information about the radius of a drop can be obtained from experimental data on its motion in the absence of an electrostatic field.; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> g; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and given that; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> m - m; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> = 4; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> /; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> πr; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> (; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ - ρ; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">); font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">;
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> where; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ρ; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - the density of the oil drop, from (3) we get:
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> (10)
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> In his experiments, Millikan changed the charge of the drop by bringing a piece of radium to the condenser. ...; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 1), as a result of which the drop could capture an additional positive or negative charge. it is clear that with a greater probability it will attach to itself positive ions. On the other hand, the addition of negative ions is not excluded. In both cases, the charge of the drop will change and -; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> abruptly; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - the speed of its movement; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> v; font-family: "Arial"; vertical-align: super "xml: lang =" en-US "lang =" en-US "> I; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> E; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">.; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> q; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> the changed drop charge in accordance with (5) is determined by the ratio:
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> q; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 0; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> = (; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: super "xml: lang =" en-US "lang =" en-US "> I; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> +; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> g; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">); font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> k; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> /; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (11)
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> From (5) and (11) the value of the charge attached by the drop is determined:
; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> Δ; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> q; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> =; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> q; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> q; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> 0; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> =; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> k; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: super "xml: lang =" en-US "lang =" en-US "> I; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> -; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">) /; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> =; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> k; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> Δ; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> v; font-family: "Arial"; vertical-align: sub "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> /; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> E; font-family: "Arial" "xml: lang =" en-US "lang =" en-US "> (12)
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Comparing the charge values of the same drop, one can make sure that the change in charge and the drop charge itself are multiples of the same the same amount; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> - elementary charge. In his numerous experiments Millikan received different values of charges; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> q; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> q; font-family: "Arial"; vertical-align: sub "xml: lang =" ru-RU "lang =" ru-RU "> 0; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">, but they were always a multiple of; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> ≈; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 7 * 10; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 19; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Cl according to (1). Hence Millikan concluded that the value; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> represents the smallest amount of electricity possible in nature, that is," a portion or atom of electricity. "
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> The modern meaning of the" atom "of electricity; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">,; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 602 * 10; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: "Arial"; vertical-align: super "xml: lang =" ru-RU "lang =" ru-RU "> 19; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Cl. This quantity is the elementary electric charge carried by an electron with a negative charge; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and a proton with a charge; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">.
; font-family: "Arial"; text-decoration: underline "xml: lang =" ru-RU "lang =" ru-RU "> Note; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">:; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> subnuclear particles called" quarks have charges equal to 2/3 in modulus; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> and 1/3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">. So the quantum of electric charge should be considered 1/3; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">. But in atomic and molecular processes all charges are multiples; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> e; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">.
"xml: lang =" ru-RU "lang =" ru-RU "> Experimental installation
Millikan measured the electrical charge on spherical droplets that were formed by a spray and charged by friction against the walls of the spray. Through a hole in the upper plate of the condenser, drops fell into the space between the plates and were observed using a microscope. If the plates are not charged, the drop will fall slowly. With charged plates, the droplet motion slowed down and changed direction.
The laboratory work is fully consistent with Millikan's experience. The experience is recommended for two students. Assemble the installation as shown in Fig. 4.
; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> Connect the permanent (300; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> В; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">) and adjustable (from 0 to; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 300; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> В; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">) voltage source outputs, so that you can receive voltage within; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 300 ÷ 600; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> В; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">. Through the field direction switch, the source is connected to the Millikan apparatus. A voltmeter is connected in parallel. The optical system of the Millikan apparatus is connected to the output; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> 6,3; font-family: "Arial" "xml: lang =" en-US "lang =" en-US ">; font-family: "Arial" "xml: lang =" ru-RU "lang =" ru-RU "> В; font-family: "Arial '" xml: lang = "ru-RU" lang = "ru-RU"> voltage source.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> Fig. 4. Modern experimental setup for determining elementary charge using the Millikan device
; font-family: 'Arial'; text-decoration: underline "xml: lang =" ru-RU "lang =" ru-RU "> Pay attention; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> -; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> in the field of the microscope (Fig.; font-family: 'Arial' "xml: lang =" en-US "lang =" en-US ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 5) the image is reversed.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> Fig.; font-family: 'Arial' "xml: lang =" en-US "lang =" en-US ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 5. Oil drops (white dots) between the condenser plates. The distance between the graduation glass marks in the eyepiece field is 0.029; font-family: 'Arial' "xml: lang =" en-US "lang =" en-US ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> mm.
"xml: lang =" uk-UA "lang =" uk-UA "> Control"xml: lang =" ru-RU "lang =" ru-RU "> th"xml: lang =" uk-UA "lang =" uk-UA ">"xml: lang =" ru-RU "lang =" ru-RU "> questions"xml: lang =" uk-UA "lang =" uk-UA ">"xml: lang =" ru-RU "lang =" ru-RU "> and"xml: lang =" uk-UA "lang =" uk-UA "> set"xml: lang =" ru-RU "lang =" ru-RU "> and"xml: lang =" uk-UA "lang =" uk-UA "> i
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 1. Formulate the law of discreteness of the charge.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 2. Formulate Stokes's law.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 3. What is the physical meaning of viscosity η? From which physical law can we get its dimension?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 4. What forces act on a drop in Millikan's experiment?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 5. How to calculate the force acting on a charged particle in the electric field of a capacitor?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 6. Why in this experiment can the droplet speed be considered constant?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 7. Why is the air in the condenser exposed to X-rays, ultraviolet rays or radiation from radioactive drugs?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 8. Why does the steady-state droplet velocity change by a specific value during irradiation?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 9. Get formula (6).
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 10. Get formula (7).
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 11. Why, when irradiated, a drop can capture a charge of the same sign as its own charge, because charges of the same name are repelled? Does the frequency of capture by a drop of the same charge depend on temperature, on the charge of the drop, on the charge of the captured ion?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 12. Why can't you measure the radius of a drop directly with a microscope?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 13. Stokes formula; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> F; font-family: 'Arial' "xml: lang =" en-US "lang =" en-US ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> =; font-family: 'Arial' "xml: lang =" en-US "lang =" en-US ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 6πη; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> rv; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> not applicable if the radius of the drop is less than the free path of molecules; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> λ; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU ">. Estimate the mean free path at atmospheric pressure and room temperature. After calculating the droplet radius from the experimental data, assess whether the condition is satisfied that the radius of the drop; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> r; font-family: 'Arial' "xml: lang =" en-US "lang =" en-US ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU ">>>; font-family: 'Arial' "xml: lang =" en-US "lang =" en-US ">; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> λ; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> (that is, the Stokes formula is applicable and data processing by formulas (5 and 11) is acceptable.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 14. Explain how to determine the elementary charge based on experimental data.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 15. Select the system of units for processing the received data and recalculate all the values of the necessary constants in this system.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 16. Using formula (5), estimate the voltage required to lift drops carrying a charge equal to 3 electron charges?
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 17. Using the Millikan method, you can determine the charge of an electron. What other methods for determining the charge of an electron do you know?
Literature
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 1; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU ">. Ioffe AF Meetings with physicists. My memories of foreign
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> physicists. L., Nauka, 1983.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 2; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU ">. Mitchell W. American Scientists and Inventors. M., Knowledge, 1975.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 3; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU ">.; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> http://www.phywe.de
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 4; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU ">. Sivukhin D.V.; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> General physics course: 5 volumes - M., 1979. - Vol. 3,“ Electricity ”.
; font-family: 'Arial' "xml: lang =" ru-RU "lang =" ru-RU "> 5.; font-family: 'Arial'; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU "> Rules for formalizing the results of experimental studies at the vison of laboratory robots in the course" Primary physics ". Vorobyova N. V., Gorchinsky O.D., Kovalenko V.F., 2004.; font-family: 'Arial'; color: # 000000 "xml: lang =" ru-RU "lang =" ru-RU ">
Order work today with a discount of up to 25%
Is free
Find out the cost of work
Parshina Anna, Sevalnikov Alexey, Luzyanin Roman.
Purpose of work: learn how to determine the value of an elementary charge by electrolysis; to examine charge determination methods electron.
Equipment: cylindrical vessel with a solution of copper sulfate, lamp, electrodes, scales, ammeter, constant voltage source, rheostat, clock, key, connecting wires.
Download:
Preview:
To use the preview of presentations, create yourself a Google account (account) and log into it: https://accounts.google.com
Slide captions:
Laboratory work Determination of elementary charge by electrolysis The students of the 10th grade Chuchkovskaya secondary school: Parshina Anna, Sevalnikov Aleksey, Luzyanin Roman. Head: physics teacher Chekalina O.Yu.
Purpose of the work: to learn how to determine the value of an elementary charge by the electrolysis method; study methods for determining the charge of an electron. Equipment: a cylindrical vessel with a solution of copper sulfate, a lamp, electrodes, scales, ammeter, constant voltage source, rheostat, clock, key, connecting wires.
We have assembled the chain: Progress:
The result of our work
We learned how to determine the value of an elementary charge by the electrolysis method, studied methods for determining the charge of an electron. Output:
V. Ya. Bryusov "The world of the electron" Perhaps these electrons are Worlds where there are five continents, Arts, knowledge, wars, thrones And the memory of forty centuries! Also, perhaps, each atom is the Universe, where there are one hundred planets; There is everything that is here, in a compressed volume, But also that which is not here. Their measures are small, but still the same Their infinity, as here; There is sorrow and passion, as here, and even there is the same world arrogance. Their wise men, putting their endless world in the center of being, Hasten to penetrate into the sparks of mystery And ponder, as I do now; And at the moment when currents of new forces are created from destruction, They scream, in dreams of self-hypnosis, That God extinguished his light!
Ministry of Education of the Russian Federation
Amur State Pedagogical University
Methods for determination of elemental electric charge
Completed by student 151g.
Venzelev A.A.
Checked by: Cheraneva T.G
Introduction.
1. Prehistory of the discovery of the electron
2. History of the discovery of the electron
3. Experiments and methods of electron discovery
3.1 Thomson's experience
3.2 Rutherford's experience
3.3. Millikan's method
3.3.1. short biography
3.3.2. Installation Description
3.3.3. Calculation of the elementary charge
3.3.4. Conclusions from the method
3.4. Compton imaging method
Conclusion.
Introduction:
ELECTRON - the first elementary particle by the time of discovery; material carrier of the smallest mass and smallest electric charge in nature; constituent part of the atom.
The electron charge is 1.6021892. 10 -19 Cl
4.803242. 10 -10 units SGSE
The mass of an electron is 9.109534. 10 -31 kg
Specific charge e / m e 1.7588047. 10 11 cl. kg -1
The electron spin is 1/2 (in units of h) and has two projections ± 1/2; electrons obey the Fermi-Dirac statistics, fermions. They are subject to the Pauli exclusion principle.
The magnetic moment of the electron is equal to - 1.00116 m b, where m b is Bohr's magneton.
An electron is a stable particle. According to experimental data, the lifetime is t e> 2. 10 22 years old.
Does not participate in strong interactions, lepton. Modern physics considers the electron as a truly elementary particle that does not have a structure and size. If the latter are nonzero, then the electron radius r e< 10 -18 м
1.Background of the discovery
The discovery of the electron was the result of numerous experiments. By the beginning of the XX century. the existence of the electron has been established in a number of independent experiments. But, in spite of the colossal experimental material accumulated by entire national schools, the electron remained a hypothetical particle, for experience had not yet answered a number of fundamental questions. In fact, the "discovery" of the electron lasted for more than half a century and was not completed in 1897; many scientists and inventors took part in it.
First of all, there was not a single experiment in which individual electrons would participate. The elementary charge was calculated on the basis of measurements of the microscopic charge, assuming the validity of a number of hypotheses.
Uncertainty was at a crucial point. First, the electron appeared as a result of the atomistic interpretation of the laws of electrolysis, then it was discovered in a gas discharge. It was not clear whether physics was actually dealing with the same object. A large group of skeptical natural scientists believed that the elementary charge is the statistical average of charges of the most diverse magnitudes. Moreover, none of the experiments on measuring the electron charge gave strictly repeated values.
There were skeptics who generally ignored the discovery of the electron. Academician A.F. Ioffe in his memoirs about his teacher V.K. Roentgen wrote: “Until 1906 - 1907. the word electron should not have been pronounced at the Physics Institute of the University of Munich. Roentgen considered it an unproven hypothesis, often used without sufficient grounds and unnecessarily. "
The question of the mass of the electron has not been resolved; it has not been proven that charges on both conductors and dielectrics consist of electrons. The concept of "electron" did not have an unambiguous interpretation, for the experiment had not yet revealed the structure of the atom (Rutherford's planetary model would appear in 1911, and Bohr's theory in 1913).
The electron has not yet entered into theoretical constructions. The electron theory of Lorentz featured a continuously distributed charge density. In the theory of metallic conductivity, developed by Drude, it was about discrete charges, but these were arbitrary charges, on the value of which no restrictions were imposed.
The electron has not yet gone beyond the framework of "pure" science. Let us recall that the first electronic lamp appeared only in 1907. To pass from faith to conviction, it was necessary first of all to isolate the electron, to invent a method for direct and accurate measurement of the elementary charge.
The solution to this problem was not long in coming. In 1752, the idea of the discreteness of the electric charge was first expressed by B. Franklin. The discreteness of charges was experimentally substantiated by the laws of electrolysis discovered by M. Faraday in 1834. The numerical value of an elementary charge (the smallest electric charge found in nature) was theoretically calculated on the basis of the laws of electrolysis using the Avogadro number. R. Milliken carried out a direct experimental measurement of the elementary charge in classical experiments carried out in 1908 - 1916. These experiments also provided irrefutable proof of the atomism of electricity. According to the basic concepts of electronic theory, the charge of any body arises as a result of a change in the number of electrons contained in it (or positive ions, the charge value of which is a multiple of the electron charge). Therefore, the charge of any body should change abruptly and in portions that contain an integer number of electron charges. Having established experimentally the discrete nature of the change in the electric charge, R. Millikan was able to obtain confirmation of the existence of electrons and determine the magnitude of the charge of one electron (elementary charge) using the method of oil drops. The method is based on the study of the motion of charged oil droplets in a uniform electric field of known strength E.
2.Discovery of the electron:
If we ignore what preceded the discovery of the first elementary particle - the electron, and what accompanied this outstanding event, we can say briefly: in 1897, the famous English physicist THOMSON Joseph John (1856-1940) measured the specific charge q / m cathode ray particles - "corpuscles", as he called them, by the deflection of cathode rays *) in electric and magnetic fields.
By comparing the obtained number with the specific charge of a monovalent hydrogen ion known at that time, by indirect reasoning, he came to the conclusion that the mass of these particles, later called "electrons", is much less (more than a thousand times) the mass of the lightest hydrogen ion.
In the same year, 1897, he hypothesized that electrons are an integral part of atoms, and cathode rays are not atoms or not electromagnetic radiation, as some researchers of the properties of rays believed. Thomson wrote: "Thus, cathode rays represent a new state of matter, significantly different from the usual gaseous state ...; in this new state, matter is the substance from which all elements are built."
Since 1897, the corpuscular model of cathode rays began to gain general acceptance, although there was a wide variety of judgments about the nature of electricity. So, the German physicist E. Wichert believed that "electricity is something imaginary, existing only in thoughts", and the famous English physicist Lord Kelvin in the same year, 1897, wrote about electricity as a kind of "continuous fluid".
Thomson's idea of cathode-ray corpuscles as the main components of the atom was not met with great enthusiasm. Some of his colleagues thought he was mystifying them when he suggested that the particles of the cathode rays should be considered as possible components of the atom. The true role of Thomson corpuscles in the structure of the atom could be understood in combination with the results of other studies, in particular, with the results of the analysis of spectra and the study of radioactivity.
On April 29, 1897, Thomson delivered his famous message at a meeting of the Royal Society of London. The exact time of the discovery of the electron - day and hour - cannot be named in view of its originality. This event was the result of many years of work by Thomson and his collaborators. Neither Thomson nor anyone else ever observed an electron in the literal sense, no one was able to isolate an individual particle from a beam of cathode rays and measure its specific charge. The author of the discovery is J.J. Thomson because his ideas about the electron were close to modern ones. In 1903 he proposed one of the first models of the atom - "pudding with raisins", and in 1904 he suggested that electrons in an atom are divided into groups, forming various configurations that determine the periodicity of chemical elements.
The place of discovery is precisely known - the Cavendish Laboratory (Cambridge, Great Britain). Created in 1870 by J.C. Maxwell, over the next hundred years it became the "cradle" of a whole chain of brilliant discoveries in various fields of physics, especially in atomic and nuclear. Its directors were: Maxwell J.K. - from 1871 to 1879, Lord Rayleigh - from 1879 to 1884, Thomson J.J. - from 1884 to 1919, Rutherford E. - from 1919 to 1937, Bragg L. - from 1938 to 1953; Deputy Director in 1923-1935 - Chadwick J.
Scientific experimental research was carried out by one scientist or a small group in an atmosphere of creative research. Laurence Bragg later recalled his work in 1913 with his father, Henry Bragg: “It was a wonderful time when new exciting results were received almost every week, like the discovery of new gold-bearing areas where nuggets can be picked up right from the ground. the beginning of the war *), which ended our joint work. "
3. Methods of opening an electron:
3.1 Thomson's experience
Joseph John Thomson, 1856-1940
English physicist, better known simply as J.J. Thomson. Born in Cheetham Hill, a suburb of Manchester, the son of a second-hand bookseller and antiquarian. In 1876 he won a scholarship to study at Cambridge. In 1884-1919 he was a professor at the Department of Experimental Physics at the University of Cambridge and concurrently - the head of the Cavendish Laboratory, which, through Thomson's efforts, became one of the most famous research centers in the world. At the same time in 1905-1918 he was a professor at the Royal Institute in London. Laureate of the Nobel Prize in Physics in 1906 with the formulation "for the study of the passage of electricity through gases", which, of course, includes the discovery of the electron. Thomson's son George Paget Thomson (1892-1975) also eventually became a Nobel laureate in physics - in 1937 for the experimental discovery of electron diffraction by crystals.
In 1897, the young English physicist J.J. Thomson became famous for centuries as the discoverer of the electron. In his experiment, Thomson used an improved cathode-ray tube, the design of which was supplemented with electric coils that created (according to Ampere's law) a magnetic field inside the tube, and a set of parallel electric capacitor plates that created an electric field inside the tube. This made it possible to study the behavior of cathode rays under the influence of both magnetic and electric fields.
Using a new tube design, Thomson has consistently shown that: (1) cathode rays are deflected in a magnetic field in the absence of an electric one; (2) cathode rays are deflected in an electric field in the absence of a magnetic one; and (3) under the simultaneous action of electric and magnetic fields of balanced intensity, oriented in directions that separately cause deflections in opposite directions, the cathode rays propagate rectilinearly, that is, the action of the two fields is mutually balanced.
Thomson found that the relationship between electric and magnetic fields, at which their action is balanced, depends on the speed with which the particles move. Through a series of measurements, Thomson was able to determine the speed of movement of the cathode rays. It turned out that they move much slower than the speed of light, from which it followed that cathode rays can only be particles, since any electromagnetic radiation, including light itself, propagates at the speed of light (see Spectrum of electromagnetic radiation). These are unknown particles. Thomson called "corpuscles", but soon they began to be called "electrons".
It immediately became clear that electrons must exist in the composition of atoms - otherwise, where would they come from? April 30, 1897 - the date Thomson reported his results at a meeting of the Royal Society of London - is considered the birthday of the electron. And on this day, the idea of the "indivisibility" of atoms became a thing of the past (see Atomic theory of the structure of matter). Together with the discovery of the atomic nucleus that followed a little over ten years later (see Rutherford's experiment), the discovery of the electron laid the foundation for the modern model of the atom.
The above described "cathode", or rather, cathode-ray tubes became the simplest predecessors of modern television kinescopes and computer monitors, in which strictly controlled amounts of electrons are knocked out from the surface of a hot cathode, under the influence of variable magnetic fields deflect at strictly specified angles and bombard the phosphorescent cells of screens , forming on them a clear image resulting from the photoelectric effect, the discovery of which would also be impossible without our knowledge of the true nature of the cathode rays.
3.2 Rutherford's experience
Ernest Rutherford, First Baron Rutherford of Nelson, 1871-1937
New Zealand physicist. Born in Nelson, the son of an artisan farmer. Won a scholarship to study at the University of Cambridge in England. After graduation, he was assigned to the Canadian McGill University, where, together with Frederick Soddy (1877-1966), he established the basic laws of the phenomenon of radioactivity, for which he was awarded the Nobel Prize in Chemistry in 1908. Soon the scientist moved to the University of Manchester, where, under his leadership, Hans Geiger (1882-1945) invented his famous Geiger counter, began researching the structure of the atom and in 1911 discovered the existence of the atomic nucleus. During the First World War, he was engaged in the development of sonars (acoustic radars) to detect enemy submarines. In 1919 he was appointed professor of physics and director of the Cavendish Laboratory at Cambridge University and in the same year discovered nuclear decay as a result of bombardment with high-energy heavy particles. In this post, Rutherford remained until the end of his life, while serving for many years as President of the Royal Scientific Society. Buried in Westminster Abbey next to Newton, Darwin and Faraday.
Ernest Rutherford is a unique scientist in the sense that he made his main discoveries after receiving the Nobel Prize. In 1911, he succeeded in an experiment that not only allowed scientists to look deep into the atom and get an idea of its structure, but also became a model of grace and depth of design.
 Using a natural source of radioactive radiation, Rutherford built a cannon that produced a directed and focused stream of particles. The gun was a lead box with a narrow slot, inside which radioactive material was placed. Due to this, the particles (in this case, alpha particles, consisting of two protons and two neutrons) emitted by the radioactive substance in all directions, except for one, were absorbed by the lead screen, and only a directed beam of alpha particles flew out through the slot.
Using a natural source of radioactive radiation, Rutherford built a cannon that produced a directed and focused stream of particles. The gun was a lead box with a narrow slot, inside which radioactive material was placed. Due to this, the particles (in this case, alpha particles, consisting of two protons and two neutrons) emitted by the radioactive substance in all directions, except for one, were absorbed by the lead screen, and only a directed beam of alpha particles flew out through the slot.
|
given direction. As a result, a perfectly focused beam of alpha particles flew up to the target, and the target itself was the thinnest sheet of gold foil. The alpha ray hit her. After colliding with the foil atoms, the alpha particles continued their path and hit a luminescent screen installed behind the target, on which flashes were recorded when alpha particles hit it. From them, the experimenter could judge in what quantity and how much alpha particles deviate from the direction of rectilinear motion as a result of collisions with foil atoms.
Rutherford, however, noticed that none of his predecessors even tried to test experimentally whether some alpha particles were deflected at very large angles. The raisin grid model simply did not allow the existence of such dense and heavy structural elements in the atom that they could deflect fast alpha particles at significant angles, so no one bothered to test this possibility. Rutherford asked one of his students to re-equip the installation in such a way that it was possible to observe the scattering of alpha particles at large deflection angles - just to clear his conscience, to completely eliminate this possibility. The detector was a sodium sulfide-coated screen, a material that gives off a fluorescent flash when an alpha particle hits it. Imagine the surprise not only of the student directly conducting the experiment, but also of Rutherford himself when it turned out that some particles are deflected at angles up to 180 °!
The picture of the atom, drawn by Rutherford based on the results of the experiment, is well known to us today. An atom consists of a superdense, compact nucleus that carries a positive charge, and negatively charged light electrons around it. Later, scientists have provided a reliable theoretical basis for this picture (see Bora Atom), but it all started with a simple experiment with a small sample of radioactive material and a piece of gold foil.
3.2 Method Milliken
3.2.1. Short biography:
Robert Millikan was born in 1868 in Illinois to a poor priestly family. He spent his childhood in the provincial town of Macvocket, where much attention was paid to sports and poorly taught. A high school principal who taught physics, said, for example, to his young listeners: “How can you make sound out of waves? Nonsense, boys, it's all nonsense! "
Oberdeen College was no better, but Millikan, who had no financial support, had to teach physics in high school himself. In America then there were only two textbooks on physics, translated from French, and the talented young man did not have any difficulties to study them and successfully teach. In 1893 he entered Columbia University, then went to study in Germany.
Millikan was 28 years old when he received an offer from A. Michelson to take an assistant position at the University of Chicago. At the beginning, he was engaged here almost exclusively in pedagogical work and only at the age of forty began scientific research, which brought him worldwide fame.
3.2.2. First experiences and problem solving:
The first experiments boiled down to the following. Between the plates of the flat capacitor, to which a voltage of 4000 V was applied, a cloud was created, consisting of water droplets deposited on the ions. The top of the cloud was first observed to fall in the absence of an electric field. Then a cloud was created with the voltage turned on. The fall of the cloud took place under the influence of gravity and electrical force.
The ratio of the force acting on a drop in a cloud to the speed it acquires is the same in the first and second cases. In the first case, the force is mg, in the second mg + qE, where q is the drop's charge, E is the electric field strength. If the speed in the first case is equal to υ 1 in the second υ 2, then
Knowing the dependence of the cloud falling velocity υ on the air viscosity, we can calculate the required charge q. However, this method did not give the desired accuracy, because it contained hypothetical assumptions that were beyond the control of the experimenter.
In order to increase the accuracy of measurements, it was first of all necessary to find a way to take into account the evaporation of the cloud, which inevitably occurred during the measurement.
Reflecting on this problem, Millikan came up with the classical drop method, which opened up a number of unexpected possibilities. We will leave the story of the invention to the author himself:
“Realizing that the rate of evaporation of the droplets remained unknown, I tried to come up with a method that would completely exclude this indeterminate value. My plan was as follows. In previous experiments, the electric field could only slightly increase or decrease the speed of the fall of the top of the cloud under the influence of gravity. Now I wanted to strengthen this field so that the upper surface of the cloud remained at a constant height. In this case, it became possible to accurately determine the rate of evaporation of the cloud and take it into account in the calculations. "
To implement this idea, Millikan designed a small-sized rechargeable battery, which gave a voltage of up to 10 4 V (for that time this was an outstanding achievement of the experimenter). She had to create a field strong enough for the cloud to be held, like the "coffin of Mohammed", in limbo. “When I had everything ready,” Millikan says, and when the cloud formed, I turned the switch and the cloud was in the electric field. And at that moment it melted before my eyes, in other words, not even a small piece remained of the whole cloud, which could be observed with the help of an optical control device, as Wilson did and I was going to do. At first it seemed to me that the cloud's disappearance without a trace in the electric field between the upper and lower plates meant that the experiment ended in vain ... ”However, as often happened in the history of science, failure gave rise to a new idea. She led to the famous drop method. “Repeated experiments,” writes Millikan, “have shown that after the cloud dissipates in a powerful electric field, in its place several separate water droplets could be discerned"(Emphasized by me. - V. D.). The "unsuccessful" experiment led to the discovery of the possibility of keeping in equilibrium and observing individual droplets for a sufficiently long time.
But during the observation period, the mass of the water droplet changed significantly as a result of evaporation, and Millikan, after many days of searching, switched to experiments with oil droplets.
The experimental procedure turned out to be simple. A cloud is formed between the condenser plates by adiabatic expansion. It consists of droplets with charges of different magnitude and sign. When the electric field is turned on, drops with charges of the same name as the charge on the upper plate of the capacitor quickly fall, and drops with the opposite charge are attracted by the upper plate. But a certain number of drops have such a charge that the force of gravity is balanced by the electrical force.
After 7 or 8 minutes. the cloud dissipates, and a small number of drops remain in the field of view, the charge of which corresponds to the indicated balance of forces.
Millikan observed these drops as distinct bright points. “The history of these drops usually proceeds like this,” he writes. “In the case of a slight predominance of gravity over the force of the field, they begin to slowly fall, but since they gradually evaporate, their downward movement soon ceases, and they become motionless for quite a long time. ... Then the field begins to prevail and the drops begin to rise slowly. At the end of their life in the space between the plates, this upward movement becomes very strongly accelerated, and they are attracted at high speed to the upper plate. "
3.2.3. Installation description:
The diagram of the Millikan apparatus, with the help of which decisive results were obtained in 1909, is shown in Figure 17.
Chamber C contained a flat capacitor made of round brass plates M and N with a diameter of 22 cm (the distance between them was 1.6 cm). A small hole p was made in the center of the top plate through which the oil droplets passed. The latter were formed by blowing in a jet of oil using a spray. In this case, the air was preliminarily purified from dust by passing it through a pipe with glass wool. The oil droplets had a diameter of about 10 -4 cm.
 A voltage of 10 4 V was applied to the capacitor plates from the storage battery B. A switch could be used to short-circuit the plates and thereby destroy the electric field.
A voltage of 10 4 V was applied to the capacitor plates from the storage battery B. A switch could be used to short-circuit the plates and thereby destroy the electric field.
Oil drops falling between plates M and N were illuminated by a strong source. The behavior of the drops was observed perpendicular to the direction of the rays through the telescope.
The ions necessary for the condensation of the droplets were created by the radiation of a piece of radium 200 mg in mass located at a distance of 3 to 10 cm from the side of the plates.
With the help of a special device, the gas was expanded by lowering the piston. In 1 - 2 s after expansion, the radium was removed or obscured by a lead screen. Then the electric field was switched on and the observation of the drops into the telescope began. The pipe had a scale by which it was possible to count the distance traveled by the drop for a certain period of time. The time was recorded by an accurate watch with a lock.
In the course of observations, Millikan discovered a phenomenon that served as the key to the entire series of subsequent accurate measurements of individual elementary charges.
“While working on suspended droplets,” writes Millikan, “several times I forgot to block them from the rays of radium. Then I happened to notice that from time to time one of the drops suddenly changed its charge and began to move along the field or against it, obviously capturing in the first case a positive and in the second case a negative ion. This opened up the possibility of measuring with certainty not only the charges of individual drops, as I had done until then, but also the charge of an individual atmospheric ion.
Indeed, measuring the velocity of the same drop two times, once before and the second time after the capture of the ion, I obviously could completely exclude the properties of the drop and the properties of the medium and operate with a quantity proportional only to the charge of the captured ion. "
3.2.4. Calculation of the elementary charge:
The elementary charge was calculated by Millikan based on the following considerations. The speed of the drop is proportional to the force acting on it and does not depend on the charge of the drop.
If a drop fell between the plates of the capacitor under the action of only gravity with a velocity υ, then
When the field is turned on, directed against the force of gravity, the acting force will be the difference qE - mg, where q is the charge of the drop, E is the modulus of the field strength.
The droplet speed will be equal to:
υ 2 = k (qE-mg) (2)
If we divide equality (1) by (2), we get
![]() From here
From here
Let the drop have captured an ion and its charge has become equal to q ", and the speed of motion υ 2. The charge of this captured ion will be denoted by e.
Then e = q "- q.
Using (3), we obtain
![]()
The value is constant for a given drop.
3.2.5. Conclusions from the Millikan method
Consequently, any charge captured by the drop will be proportional to the difference in velocities (υ "2 - υ 2), in other words, proportional to the change in the droplet velocity due to the capture of an ion! Numerous observations have shown the validity of formula (4). It turned out that the value of e can only change in jumps! Charges e, 2e, 3e, 4e, etc. are always observed.
“In many cases,” writes Millikan, “the drop was observed for five or six hours, and during this time it captured not eight or ten ions, but hundreds of them. In total, I observed the capture of many thousands of ions in this way, and in all cases the captured charge ... was either exactly equal to the smallest of all captured charges, or it was equal to a small integer multiple of this value. This is a direct and irrefutable proof that the electron is not a "statistical average", but that all electric charges on the ions are either exactly equal to the charge of the electron, or represent small integer multiples of this charge. "
So, atomism, discreteness, or, in modern terms, the quantization of an electric charge has become an experimental fact. Now it was important to show that the electron is, so to speak, omnipresent. Any electric charge in a body of any nature is the sum of the same elementary charges.
Millikan's method made it possible to unequivocally answer this question. In the first experiments, charges were created by ionizing neutral gas molecules with a stream of radioactive radiation. The charge of ions captured by the droplets was measured.
When liquid is sprayed with a spray bottle, the droplets are electrified due to friction. This was well known back in the 19th century. Are these charges as quantized as the charges of the ions? Millikan "weighs" the droplets after spraying and measures the charges in the manner described above. Experience reveals the same discreteness of the electric charge.
By spraying drops of oil (dielectric), glycerin (semiconductor), mercury (conductor), Millikan proves that charges on bodies of any physical nature consist in all cases without exception of individual elementary portions of a strictly constant value. In 1913, Millikan summarizes the results of numerous experiments and gives the following value for the elementary charge: e = 4.774. 10 -10 units CGSE charge. This is how one of the most important constants of modern physics was established. Determining the electric charge has become a simple arithmetic problem.
3.4 Compton imaging method:
A large role in strengthening the idea of the reality of the electron was played by the discovery of Ch.T.R. Wilson of the effect of condensation of water vapor on ions, which led to the possibility of photographing particle tracks.
They say that A. Compton at the lecture could in no way convince a skeptical listener of the reality of the existence of microparticles. He insisted that he would believe only when he saw them with his own eyes.
Then Compton showed a photograph with an alpha-particle track, next to which was a fingerprint. "Do you know what this is?" Compton asked. “Finger,” the listener replied. "In that case," Compton declared solemnly, "this strip of light is the particle."
The photographs of the electron tracks were not only indicative of the reality of the electrons. They confirmed the assumption about the small size of the electrons and made it possible to compare with experiment the results of theoretical calculations, in which the electron radius appeared. The experiments, which were initiated by Lenard in the study of the penetrating ability of cathode rays, showed that very fast electrons emitted by radioactive substances give tracks in the gas in the form of straight lines. The track length is proportional to the electron energy. Photographs of high-energy alpha-particle tracks show that the tracks are composed of a large number of points. Each point is a water droplet appearing on an ion, which is formed as a result of the collision of an electron with an atom. Knowing the size of the atom and their concentration, we can calculate the number of atoms through which the α-particle must pass at a given distance. A simple calculation shows that an α-particle must pass about 300 atoms before it meets one of the electrons that make up the atomic shell on its way and ionizes.
This fact convincingly indicates that the volume of electrons is a negligible fraction of the volume of an atom. The track of a low-energy electron is curved; therefore, a slow electron is deflected by an intra-atomic field. It produces more ionization acts on its way.
From scattering theory, data can be obtained to estimate the deflection angles as a function of the electron energy. These data are well confirmed by the analysis of real tracks. The coincidence of theory with experiment has strengthened the concept of the electron as the smallest particle of matter.
Conclusion:
Measurement of the elementary electric charge opened up the possibility of precise determination of a number of the most important physical constants.
Knowing the value of e automatically makes it possible to determine the value of the fundamental constant - Avogadro's constant. Before Millikan's experiments, there were only rough estimates of the Avogadro constant, which were given by the kinetic theory of gases. These estimates were based on calculations of the average radius of an air molecule and varied within a fairly wide range from 2. 10 23 to 20. 10 23 1 / mol.
Let us assume that we know the charge Q that has passed through the electrolyte solution and the amount of substance M that is deposited on the electrode. Then, if the charge of the ion is Ze 0 and its mass is m 0, the equality
If the mass of the deposited substance is equal to one mole,
then Q = F is the Faraday constant, and F = N 0 e, whence:
Obviously, the accuracy of determining the Avogadro constant is given by the accuracy with which the electron charge is measured. Practice demanded an increase in the accuracy of determining the fundamental constants, and this was one of the incentives to continue improving the method for measuring the quantum of electric charge. This work, which is already of a purely metrological nature, continues to this day.
The most accurate values are currently:
e = (4.8029 ± 0.0005) 10 -10. units CGSE charge;
N 0 = (6.0230 ± 0.0005) 10 23 1 / mol.
Knowing N o, it is possible to determine the number of gas molecules in 1 cm 3, since the volume occupied by 1 mole of gas is an already known constant.
Knowing the number of gas molecules in 1 cm 3, in turn, made it possible to determine the average kinetic energy of the thermal motion of a molecule. Finally, the electron charge can be used to determine the Planck constant and the Stefan-Boltzmann constant in the law of thermal radiation.
Ministry of Education of the Russian Federation
Amur State Pedagogical University
Methods for determination of elemental electric charge
Completed by student 151g.
Venzelev A.A.
Checked by: Cheraneva T.G
Introduction.
1. Prehistory of the discovery of the electron
2. History of the discovery of the electron
3. Experiments and methods of electron discovery
3.1 Thomson's experience
3.2 Rutherford's experience
3.3. Millikan's method
3.3.1. short biography
3.3.2. Installation Description
3.3.3. Calculation of the elementary charge
3.3.4. Conclusions from the method
3.4. Compton imaging method
Conclusion.
Introduction:
ELECTRON - the first elementary particle by the time of discovery; material carrier of the smallest mass and smallest electric charge in nature; constituent part of the atom.
The electron charge is 1.6021892. 10 -19 Cl
4.803242. 10 -10 units SGSE
The mass of an electron is 9.109534. 10 -31 kg
Specific charge e / m e 1.7588047. 10 11 cl. kg -1
The electron spin is 1/2 (in units of h) and has two projections ± 1/2; electrons obey the Fermi-Dirac statistics, fermions. They are subject to the Pauli exclusion principle.
The magnetic moment of the electron is equal to - 1.00116 m b, where m b is Bohr's magneton.
An electron is a stable particle. According to experimental data, the lifetime is t e> 2. 10 22 years old.
Does not participate in strong interactions, lepton. Modern physics considers the electron as a truly elementary particle that does not have a structure and size. If the latter are nonzero, then the electron radius r e< 10 -18 м
1.Background of the discovery
The discovery of the electron was the result of numerous experiments. By the beginning of the XX century. the existence of the electron has been established in a number of independent experiments. But, in spite of the colossal experimental material accumulated by entire national schools, the electron remained a hypothetical particle, for experience had not yet answered a number of fundamental questions. In fact, the "discovery" of the electron lasted for more than half a century and was not completed in 1897; many scientists and inventors took part in it.
First of all, there was not a single experiment in which individual electrons would participate. The elementary charge was calculated on the basis of measurements of the microscopic charge, assuming the validity of a number of hypotheses.
Uncertainty was at a crucial point. First, the electron appeared as a result of the atomistic interpretation of the laws of electrolysis, then it was discovered in a gas discharge. It was not clear whether physics was actually dealing with the same object. A large group of skeptical natural scientists believed that the elementary charge is the statistical average of charges of the most diverse magnitudes. Moreover, none of the experiments on measuring the electron charge gave strictly repeated values.
There were skeptics who generally ignored the discovery of the electron. Academician A.F. Ioffe in his memoirs about his teacher V.K. Roentgen wrote: “Until 1906 - 1907. the word electron should not have been pronounced at the Physics Institute of the University of Munich. Roentgen considered it an unproven hypothesis, often used without sufficient grounds and unnecessarily. "
The question of the mass of the electron has not been resolved; it has not been proven that charges on both conductors and dielectrics consist of electrons. The concept of "electron" did not have an unambiguous interpretation, for the experiment had not yet revealed the structure of the atom (Rutherford's planetary model would appear in 1911, and Bohr's theory in 1913).
The electron has not yet entered into theoretical constructions. The electron theory of Lorentz featured a continuously distributed charge density. In the theory of metallic conductivity, developed by Drude, it was about discrete charges, but these were arbitrary charges, on the value of which no restrictions were imposed.
The electron has not yet gone beyond the framework of "pure" science. Let us recall that the first electronic lamp appeared only in 1907. To pass from faith to conviction, it was necessary first of all to isolate the electron, to invent a method for direct and accurate measurement of the elementary charge.
The solution to this problem was not long in coming. In 1752, the idea of the discreteness of the electric charge was first expressed by B. Franklin. The discreteness of charges was experimentally substantiated by the laws of electrolysis discovered by M. Faraday in 1834. The numerical value of an elementary charge (the smallest electric charge found in nature) was theoretically calculated on the basis of the laws of electrolysis using the Avogadro number. R. Milliken carried out a direct experimental measurement of the elementary charge in classical experiments carried out in 1908 - 1916. These experiments also provided irrefutable proof of the atomism of electricity. According to the basic concepts of electronic theory, the charge of any body arises as a result of a change in the number of electrons contained in it (or positive ions, the charge value of which is a multiple of the electron charge). Therefore, the charge of any body should change abruptly and in portions that contain an integer number of electron charges. Having established experimentally the discrete nature of the change in the electric charge, R. Millikan was able to obtain confirmation of the existence of electrons and determine the magnitude of the charge of one electron (elementary charge) using the method of oil drops. The method is based on the study of the motion of charged oil droplets in a uniform electric field of known strength E.
2.Discovery of the electron:
If we ignore what preceded the discovery of the first elementary particle - the electron, and what accompanied this outstanding event, we can say briefly: in 1897, the famous English physicist THOMSON Joseph John (1856-1940) measured the specific charge q / m cathode ray particles - "corpuscles", as he called them, by the deflection of cathode rays *) in electric and magnetic fields.
By comparing the obtained number with the specific charge of a monovalent hydrogen ion known at that time, by indirect reasoning, he came to the conclusion that the mass of these particles, later called "electrons", is much less (more than a thousand times) the mass of the lightest hydrogen ion.
In the same year, 1897, he hypothesized that electrons are an integral part of atoms, and cathode rays are not atoms or not electromagnetic radiation, as some researchers of the properties of rays believed. Thomson wrote: "Thus, cathode rays represent a new state of matter, significantly different from the usual gaseous state ...; in this new state, matter is the substance from which all elements are built."
Since 1897, the corpuscular model of cathode rays began to gain general acceptance, although there was a wide variety of judgments about the nature of electricity. So, the German physicist E. Wichert believed that "electricity is something imaginary, existing only in thoughts", and the famous English physicist Lord Kelvin in the same year, 1897, wrote about electricity as a kind of "continuous fluid".
Thomson's idea of cathode-ray corpuscles as the main components of the atom was not met with great enthusiasm. Some of his colleagues thought he was mystifying them when he suggested that the particles of the cathode rays should be considered as possible components of the atom. The true role of Thomson corpuscles in the structure of the atom could be understood in combination with the results of other studies, in particular, with the results of the analysis of spectra and the study of radioactivity.
On April 29, 1897, Thomson delivered his famous message at a meeting of the Royal Society of London. The exact time of the discovery of the electron - day and hour - cannot be named in view of its originality. This event was the result of many years of work by Thomson and his collaborators. Neither Thomson nor anyone else ever observed an electron in the literal sense, no one was able to isolate an individual particle from a beam of cathode rays and measure its specific charge. The author of the discovery is J.J. Thomson because his ideas about the electron were close to modern ones. In 1903 he proposed one of the first models of the atom - "pudding with raisins", and in 1904 he suggested that electrons in an atom are divided into groups, forming various configurations that determine the periodicity of chemical elements.
The place of discovery is precisely known - the Cavendish Laboratory (Cambridge, Great Britain). Created in 1870 by J.C. Maxwell, over the next hundred years it became the "cradle" of a whole chain of brilliant discoveries in various fields of physics, especially in atomic and nuclear. Its directors were: Maxwell J.K. - from 1871 to 1879, Lord Rayleigh - from 1879 to 1884, Thomson J.J. - from 1884 to 1919, Rutherford E. - from 1919 to 1937, Bragg L. - from 1938 to 1953; Deputy Director in 1923-1935 - Chadwick J.
Scientific experimental research was carried out by one scientist or a small group in an atmosphere of creative research. Laurence Bragg later recalled his work in 1913 with his father, Henry Bragg: “It was a wonderful time when new exciting results were received almost every week, like the discovery of new gold-bearing areas where nuggets can be picked up right from the ground. the beginning of the war *), which ended our joint work. "
3. Methods of opening an electron:
3.1 Thomson's experience
Joseph John Thomson, 1856-1940
English physicist, better known simply as J.J. Thomson. Born in Cheetham Hill, a suburb of Manchester, the son of a second-hand bookseller and antiquarian. In 1876 he won a scholarship to study at Cambridge. In 1884-1919 he was a professor at the Department of Experimental Physics at the University of Cambridge and concurrently - the head of the Cavendish Laboratory, which, through Thomson's efforts, became one of the most famous research centers in the world. At the same time in 1905-1918 he was a professor at the Royal Institute in London. Laureate of the Nobel Prize in Physics in 1906 with the formulation "for the study of the passage of electricity through gases", which, of course, includes the discovery of the electron. Thomson's son George Paget Thomson (1892-1975) also eventually became a Nobel laureate in physics - in 1937 for the experimental discovery of electron diffraction by crystals.