The burning reaction. Types of burning. Combustion characteristics. Speed \u200b\u200bburning of solid combustible substances and liquids
Study of the combustion rate of high-energy mixture solid fuels
Introduction
1. Literary review
1.1. Rocket fuel
1.1.2 Colloid fuel
1.1.3 Mixed fuel
1.1.4 Physical Properties
1.1.5 Gore mechanism
1.1.6 Fuel combustion rate
1.1.7 Elementary composition. Conditional chemical formula
2. Methods of experiment
2.2 Methods of manufacturing samples.
3. Experimental data
Bibliography
Introduction
The combustion of explosives is used in practice has long since the invention of black powder. However, the patterns of combustion of explosives and powders at constant pressure are examined relatively recently: the first work in this area began to be launched by K. Andreev and A.F. Belyaev in the 1930s of our century and treated the field of pressures not exceeding 100-150 atm.
There is still no sufficient clarity in what factors the dependence of the combustion rate of ballistic and mix powders is determined from pressure, as it depends on their composition, as it is associated with the corresponding dependencies of individual explosives that are included in their composition and, finally, the combustion rate is associated. with chemical structures of explosives. In this regard, the study of the process of burning explosives is of great interest.
The main objective of this monograph is to systematize and summarize new experimental and theoretical data on the effect of positive and negative catalysts for the combustion of individual explosives of various classes.
One of the ways to increase the energy capabilities of mixed fuels is the use of metal in their form of powders.
Aluminum powder remains one of the main components of solid fuels. For fine aluminum, a significant increase in the combustion rate was detected, which may be associated with the complete combustion of these metal fractions in the air.
All the studied compositions were characterized by a constant coefficient of excess oxidizing agent, equal to 0.9.
In addition to burning, the production of burning was determined.
1. Literary review
1.1 Rocket fuel
In rocket engines on chemical fuel, the emission of energy occurs as a result chemical reaction. Energy may be allocated as a result of the following reactions:
a) the oxidation reaction (oxidation), when the energy is released during the reaction between the oxidative and combustible elements; The fuel consists in this case, at least from two substances - oxidizing agent and fuel;
b) the decomposition reaction, when heat is highlighted in the process of decomposition of a complex substance to a simpler; Fuel in this case may consist of only one substance;
c) Reactions of recombination (compound) when heat is released when connecting atoms of the same name or radicals into the molecule.
Fuel rocket engines can be divided into the following four groups: liquid fuel of separate feed, liquid unitary fuels, solid fuel, Fuel mixed aggregate state.
In the case of liquid fuel, the separation supply of energy is separated as a result of the oxidation reaction - recovery. The oxidation process can be conditionally represented as an exchange of electrons on the outer electronic shell of atoms involved in this process. In this case, the atoms of combustible elements give their electrons, and the atoms of oxidative elements acquire them.
The combustible elements include carbon C, hydrogen H, boron, aluminum AI, lithium Li, and others. The oxidative elements are fluorine F, oxygen o, chlorine Ci, Bromine Br. Fluorine and oxygen are significantly superior to other oxidative elements.
The oxidizing agent and fuel in the general case are complex compounds, which can be included in both oxidative and combustible elements, as well as neutral.
The flammable is such a substance that, regardless of whether the oxidative elements are contained in it or not, for the complete oxidation of its combustible elements requires an oxidizing agent. For example, ethyl alcohol C2H5on besides flammable elements (C and H), contains an oxidative element - oxygen, but it is completely insufficient for the complete oxidation of combustible elements of alcohol; Therefore, ethyl alcohol is flammable.
The oxidizing agent is a substance in which although there may be combustible elements, but the oxidizing elements there is significant excess in it, so that with the full oxidation of its own combustible elements that can be used to oxidize any other fuel. For example, NNO3 nitric acid or hydrogen peroxide H2O2 contain combustible element - hydrogen, however, an oxidative element (oxygen) in them is available in such a quantity that with full oxidation of hydrogen of nitric acid or hydrogen peroxide in them there remains excess oxygen that can be used for oxidation of any fuel; Therefore, NNO3 and H2O2 are oxidizers.
The shares of the oxidizing agent and fuel in the fuel are determined by the value of the ratio of components. Theoretical (stoichiometric) ratio of components æ0 is called such a minimum amount of oxidizing agent, which is necessary for complete oxidation of 1 kg fuel. In other words, the theoretical ratio of components, this is the ratio of the costs of the oxidizing agent and fuel, in which the oxidizing agent is completely oxidizing the fuel, without remaining in the excess.
The actual ratio of components æ is the actual ratio of the costs of the oxidizing agent and fuel supplied to the chamber, which may differ from the theoretical.
Usually æ.<æ0.
The ratio α \u003d æ / æ0 is called an excess oxidant coefficient. The coefficient of excess of the oxidant, in which the maximum value of the specific traction is called optimal.
Separate feed fuel can be self-igniting and non-flameless. The first includes such fuels, the ignition of which begins in itself during the contact of the oxidizing agent and fuel in the conditions available in the chamber at startup, without any additional intervention. Non-flameless fuels for primary ignition (when starting the engine) require ignition tools.
A mixture of oxidizing agent and fuel in general is explosive.
Unitary (single-component) fuel can be such an individual substance or such a pre-prepared mixture of substances, which, under certain conditions, heat as a result of chemical reactions of decomposition or oxidation; In the latter case, all the elements needed for oxidation are in the unitary fuel itself.
Solid rocket fuels are naturally unitary, as they contain in their mass all the substances necessary for the flow of a chemical reaction. The basis of solid rocket fuels can be substances capable of an exothermic reaction of decomposition, or a mixture of oxidizing agent and fuel. Solid fuels are widely used in rocket technology. They allow you to have a simple engine design and high availability to its launch. However, known solid fuels provide smaller values \u200b\u200bof specific traction than liquid.
The fuel of the mixed aggregate state consists of components located in different aggregate states; For example, liquid solid fuel in which one of the components is liquid, and the other solid. In this case, the solid component is placed in the combustion chamber, and the liquid in the tank and in some way or another is supplied to the chamber, where the chemical reaction occurs between the oxidizing agent and the flammable and the formation of gaseous combustion products.
1.1.1 Solid rocket fuels
Solid rocket fuels can be divided into two main groups: colloidal (two-axis) and blended.
1.1.2 Colloid fuel
The basis of these fuels is a nitrocletter (nitrocellulose) and the solvent, which accounts for the bulk of the fuel (more than 90%); Therefore, such fuels are called dibasic.
Nitrocellulose is obtained by treating nitric acid cellulose, the conditional formula of which [C6H7O2 (ON3)] N. At the same time, in cellulose, a number of groups are replaced by nitrate groups of ONO2. Cellulose nitrate properties depend on the number of ONO2 groups contained in them, or, which is the same, from the percentage of nitrogen; In nitrocellulose, which comes to the manufacture of solid rocket fuels, it is 12-13%. Nitrocellulose is capable of exothermic decomposition reaction; At the same time, the oxidation of combustible elements is oxidized. Nitrocellulose has a negative oxygen balance: oxygen atoms are not sufficient for complete oxidation of combustible elements. The higher the degree of cellulose nitration, i.e. The higher the nitrogen content, the more favorable oxygen balance. The heat decomposition of nitrocellulose fluctuates within 3000 - 4000kj / kg.
Nitrocellulose in pure form cannot be used as fuel due to the tendency to the explosion. By treating nitrocellulose by some solvents, a colloidal solution is obtained - a gelatin-like mass, which further processing gives high heat resistance and the form. In this form, the charges of colloidal fuels have high resistance to the explosion and the ability to uniform burning.
Nitroglycerin is most often used as a solvent. It has a higher warmth of decomposition than nitrocellulose, therefore an increase in the percentage of nitroglycerin in fuel increases the calorific value of the fuel, and therefore, the value of the specific impulse. This is also due to the fact that nitroglycerin has a positive oxygen balance, and some of the combustible elements of nitrocellulose is oxidized by redundant nitroglycerin oxygen. But the nitroglycerin content in fuels does not exceed 43%, since with the further increase in its share decreases the strength of charges and their stability deteriorates.
In addition to the main components - nitrocellulose and solvent - various additives are introduced into colloidal fuels: stabilizers that increase the stability of charges during storage, phlegmatizers, lowering fuel combustion speed, catalysts that improve the combustion process at low pressures, technological additives, facilitating the process of pressing charges, and dyes .
RDTT with dioxide fuels have specific impulses in the limits of 2000-2400n * C / kg; Large values \u200b\u200brefer to fuels with a higher content of nitroglycerin and with nitrocellulose, which has a greater degree of nitration. The density of colloid fuels lies within 1550-160 kg / m3.
1.1.3 Mixed fuel
Mixed fuels are mechanical mixtures of solid oxidizing agents and flammable.
Oxidifiers usually serve solid salts of chlorine and nitric acids rich in oxygen, in particular, ammonium perchlorate NN4CIO4, potassium perchlorate KSIO4, sodium nitrate NaNo3, etc.
The main use of ammonium perchlorate was the main use of mixed fuel. Its use allows you to get fuel with acceptable operational and fairly high energy characteristics. Potassium perchlorate, despite the large content of active oxygen, provides a smaller value of the specific impulses due to the formation of solid KSI combustion products.
Nitrates - sodium, ammonia and potassium nitrates - cheap affordable products, but they are less effective than perchlorates, and hygroscopic and therefore, as well as perchlorate potassium, there are no wide practical application.
Fuel in mixed fuels also performs the role of a bundle. As combustible in these fuels, substances are used with sufficiently high calorific value and can bind individual fuel components. Usually, synthetic rubber type polymers, resins and plastics (for example, polyurethanes, polybutadienes, polysulfides) are used for these purposes.
Solid mixture fuels are manufactured by introducing chopped oxidizer particles into a molten fuel - a ligament. The mass thus obtained is either used for the manufacture of checkers, which are then inserted into the combustion chamber, or poured directly into the combustion chamber, where it hardens and firmly connects with the walls. The fuel charge should be sufficiently elastic to be under the action of thermal stresses caused by different coefficients of linear expansion of fuel materials and the chamber, cracks were not formed in it. The use of charges, firmly related design, improves the useful use of the camera volume; In addition, if the charge burning comes from the center to the periphery, eliminates the need to protect the walls of the combustion chamber with thermal insulating materials.
For most combinations of solid combustible and oxidizing agents in a stoichiometric mixture, an oxidizer accounts for 85-90% or more. However, with its considerable content due to the low fraction of fuel - the ligaments deteriorate the mechanical properties of charges. Therefore, usually in the mixture fuels, the excess coefficient is less than a unit and below the optimal value. From this point of view, combinations that have a relatively smaller value of æ0 are more favorable.
Swimming fuels without additives provide specific impulses of the same order as the two-axis; The density of the mixture fuel is in the range of 1700-1800 kg / m3. Enhance the specific impulse can be achieved if you enter a certain amount of metallic fuel. Currently, mix fuels containing aluminum powder additives are applied, which increases the calorific value of the fuel. True, in the combustion products, a polyhydric oxide of aluminum AI2O3 appears, a significant part of which is condensed; Nevertheless, there is a winnings in a specific impulse. Aluminum additives up to 5-15% increase the specific impulse per 100-200 H * C / kg. Other ways of increasing the specific pulse of solid fuels, in particular, the synthesis of combustible, in which metal elements are chemically related to other components are being developed. An increase in the specific impulse is also possible to use more efficient oxidizing agents. This, in particular, is Licio4 lithium perchlorate. Increasing the rust of the oxidant in solid mixed fuels to certain limits should also contribute to the increase in the specific impulse.
Mixed fuels have a number of advantages over a two-friendly. They are cheaper, more technological, allow you to create charges, tightly adjacent to the shell; In the presence of metal additives, they provide a greater specific impulse; Finally, they allow by changing the recipe to obtain a wider range of changing fuel properties.
Sometimes solid mixed-type fuels are used, including elements of both mixture and dibasic fuels. For example, we specify the composition of the engine fuel by one of the ballistic missiles; Ammonium perchlorate, nitroglycerin, nitrocellulose, aluminum powder.
Despite the diversity of the existing and developed in foreign laboratories, mix fuels, as a rule, contain the following substances (by weight):
Oxidifiers (perchlorate potassium, ammonium nitrate) .................. ..60-80%
Furi-binders (rubbers, polyurethanes) ............ 25-15%
Aluminum (in the form of powder) ............................................. 10-5%
Catalysts and other special substances ......................To 5%.
Ammonium nitrate (ammonium nitrate) NH4NO3- white crystalline powder with a specific weight of 1.7g / cm3. Decomposes when heated above 170 ° C. Very hygroscopic. Can burn and explode. When combustion, a large number of gaseous products are allocated.
1.1.4 Physical Properties
The fuel density is responsible for their characteristic and is always monitored in the production of fuels.
The reduced fuel density indicates that there are pores and emptiness in the fuel, unacceptable for high-quality charges of fuels. The reduced density affects the fuel combustion rate: with a decrease in density, it increases and vice versa.
The thermal characteristics include the specific heat capacity of the CP, the thermal conductivity coefficient λ and the temperature coefficient α. These quantities characterize the ability of fuels to perceive heat when exposed to temperature and carry out (distribute) in the thickness of the fuel. They are used in theoretical calculations of thermal voltages of charges bonded with the engine chamber, fuel combustion rates in engines.
Changing the physical properties of fuels during storage occurs under the influence of changes in the external temperature, moisture and time.
On the surface of ultrafine particles there is a radical restructuring of the arrangement of atoms and changes in the type of interatomic bonds compared to the surface of large particles ..
In ultrafine particles, a special type of long-range is implemented, in which the interatomic distances are naturally changed during the transition from the center of the particle to its surface, which leads to the formation of a plurality of defects both on the particle surface and in its volume and increases the activity of such a system as a whole.
1.1.5 Gore mechanism
In the mechanism of combustion of mixed fuels, there are a number of features determined by the composition and nature of these substances.
The combustion of mixed fuels begins in the solid phase from the thermal decay of oxidizing agents and fuel-binding substances. The combustion process is completed in the gas phases due to intense chemical reactions between the gaseous products of the thermal decay of the components.
For combustion of mixed fuels, large temperature of the combustion surface (up to 500-600 ° C) and the maximum combustion temperatures are most characteristic of the combustion surface.
The process of combustion of solid rocket fuels is very sensitive to external influences - pressure and initial fuel temperature. When the pressure and temperature increases, dark and mixed zones are sharply reduced, and the flame zone approaches the surface of the burning. The heat supply to the burning surface increases, the burning rate is growing, and the heating zone is narrowed. To avoid these adverse conditions, combustion catalysts, accelerating chemical reactions in solid and gas phases, which contribute to more complete burning and ultimately improve the characteristics of the fuel.
The introduction of AI in fuel systems containing an organic fuel and an inorganic oxidizer contributes to increasing flammability, combustion rate and affects the dependence of the burning rate from pressure.
1.1.6 Fuel combustion rate
For a quantitative estimate of the combustion process, either the speed of moving the combustion front is used, or a mass of fuel that burns per unit of time from a surface unit.
In the first case, the combustion rate is called linear and expressed in mm / s or cm / s, in the second - mass and expressed in g / cm2 * sec. In practice, more often use linear combustion rate.
The combustion rate is a very important feature characteristic of fuel, as it is judged by the number of gases that are formed during the burning of fuel per unit of time from the charge surface. It is one of the main parameters when designing fuels charges.
The burning rate of fuel depends on the pressure in the engine, the initial temperature of the fuel, its density, energy characteristics, the nature of the components of the fuel, the size of the oxidant particles (in mixed fuels) and combustion catalysts.
For practical purposes, it is always necessary to know, first of all, the dependence of the burning rate from pressure.
The dependence of the combustion rate of solid fuels from pressure is determined by an experimental way and expressed by the formulas that the names of the laws of combustion. The law of burning rate is experimental for each fuel in the desired pressure range.
1.1.7 Elementary composition
Conditional chemical formula.
The composition of the substance in the mass fractions of individual elements is called the elementary composition. The general formula for the mass fraction of a separate (k-th) element in the substance has the form:
;here bk is the mass fraction of the k-th element;
aK - the number of atoms of this element in the molecule of the compound under consideration;
AK- atomic weight of this element;
If you restrict ourselves to the elements H, C, N and O, in the general case, the chemical formula of the substance has the form
Then the elementary composition will be
; bh \u003d; bo \u003d; BN \u003d.Here μ \u003d 12m + n + 16p + 14q is the molecular weight of the substance;
bC, BH, BO, BN - the shares of carbon, hydrogen, oxygen and nitrogen.
For carbon and hydrogen, rounded values \u200b\u200bof atomic masses were taken (μn \u003d 1, μc \u003d 12);
If the fuel or its component is a combination of several substances, then the mass fraction of a separate element is found like this:
where bk is the mass fraction of the K - th element in the mixture,
gi is the mass fraction of the individual (i-th) substance in the mixture,
bKI is the mass fraction of the K - th element in the I- M substance;
If the fuel consists of an oxidizing agent and fuel and the ratio of components æ The elementary composition of both components is known, then the mass fraction of a separate (k - go) element in the fuel will be found:
bk \u003d (bkg + ækok) / (1+).
When the components are mixtures of individual substances, then for some calculations it is convenient to use the conditional chemical formula of this component. Such formula can be built in a different way. For example, it is convenient to determine it, based on the number of atoms of various elements per 100 mass units of the component under consideration. Then the conditional chemical formula will be
where m \u003d 100bc / 12; n \u003d 100bh / 1; P \u003d 100BO / 16; q \u003d 100bn / 14,
a BC, BH, BO, BN - mass shares of the corresponding elements in this component.
2. Experiment techniques
The work used methods for the manufacture of model solid fuels, measurement of combustion rate.
2.1 Definition of the percentage of components of the fuel composition according to the known α
Knowledge of the coefficient of excess of the oxidizing agent of the system allows you to solve the inverse task, i.e. Determine the percentage composition of the fuel composition components.
Consider this on the example of fuel with α \u003d 0.90 and the content of aluminum 15 masses. %, then the content of the fuel composition can be written as
NH4NO3 - (85-x)%
Bunch - x%
Table number 1. The calculation of the equivalent formula.
| component | content, wt.% | equivalent component formula | The content of elements in fuel taking into account the mass.% | ||||||||||||||||||||||||||||||||||||||||||||||||
| Al | FROM | N. | ABOUT | N. | |||||||||||||||||||||||||||||||||||||||||||||||
| NH4NO3. | 85-H. In operation, the fuel compositions were made by manual in the laboratory technique not more than 10 grams. Mixtures for one bag. When working with fuel compositions, the following operations were carried out: weighing fuel components, mixing, formation of samples, determining their main characteristics (mass, height, diameter), reservation, re-definition of the main characteristics. During the work, the compositions containing the ammonium nitrate of the Mark of FAI, aluminum (UDP), soot and SNCI2 were manufactured. The dosage of the components was performed on electronic scales up to 0.02g. Total fuel mass 10g. A ligament (MPVT-ASP) was fully placed in a porcelain cup, and aluminum hitches (UDP), ammonium nitrate (MARKI), soot, Snci2 and hardener were prevented on tracing sheets. Then the fuel components were gradually added to the bundle and after each component, the mixture was thoroughly mixed. The hardener was introduced into the finished fuel mass, which is further stirred further. The resulting fuel mass was molded with a fluoroplastic assembly in the form of cylindrical samples with a diameter of 10 mm. The resulting samples were weighed, the height was measured, density determined. The samples were then booked on the side surface of the linoleum dissolved in acetone, and burned in air under normal conditions. 2.3 Measurement of burning speed We use a laboratory method for measuring the combustion rate. The combustion rate was determined in air at room temperature. The sample was placed on a textolite substrate. Then the charge was focused on the upper end simultaneously throughout the length of the contact border using an open flame. The combustion time was fixed by the stopwatch. The calculation of the combustion speed was carried out by the formula: U \u003d l / τ, mm / sec., Where the L - length of the sample, mm τ - combustion time, sec. To determine the combustion rate of this composition, at least three definitions were carried out. 3. Experimental data The operation used the fuel composition characterized by a constant coefficient of excess oxidant equal to 0.9. The characteristic that determines the fitness of the fuel to the study has served the density of the samples. Under normal conditions, the stationary burning rate was determined. 3.1 Characteristics of the studied batch of samples Table number 2 shows the characteristics of the studied batch of samples, namely the mass (with a booking and without), height, diameter (with booking and without), density, burning time, speed. Table number 2. Sample characteristics.
The average sample density was equal to 1.54g / cm3, and the average combustion rate of 1.15mm / s, which does not contradict the data obtained earlier in experiments with samples of the same composition. The method of calculating the component composition of the fuel mixture at α \u003d 0.9 was studied. The properties of the components of the mixed solid fuel are studied. Made samples of mixed solid fuel and their density is determined. The combustion rate of the high-energy composition is determined. Bibliography 1. TM Melkumov, N.I. Melik Pashaev, P.G. Chistyakov, A.G. Shiukov rocket engines. Moscow // Mechanical Engineering, 1976,400С. 2. I.A. Silantines, solid rocket fuels. Moscow, militial // 1964, 80 s. 3. Lidorenko N.S., Chizhik S.P., Smooth N.N. et al. Shift electronic potential in highly dispersed systems. // Izv. Academy of Sciences of the USSR. Metals. 1981. №6. from. 91-95. | ||||||||||||||||||||||||||||||||||||||||||||||||||
The main combustion characteristic is the burning rate. There is a normal (linear) and mass combustion rate.
At normal speed Understand U. n. . - a linear movement of the movement of the combustion front in the direction perpendicular surface of the combustion:
Un \u003d ℓ / τ [cm / s]
Mass combustion speedU. m. - This is the amount of the initial combustible system, burned per unit of time from the unit of the surface of the combustion front.
Um \u003d Un × P0 [g / cm2 × s]
Speaking of burning speed, it is necessary to distinguish between the combustion itself and the surface spread of burning. When burning the powder column, the condensed phase usually takes the form of a cone, while the combustion surface turns out to be much more source.
(The cutting of combustible products of incomplete combustion due to oxygen leads to an increase in temperature and, as a result, causes acceleration of the spread of burning over the charge surface).
When burning the powder elements in the barrel channel, the ignition usually occurs throughout the free surface.
The weight number of gases formed during combustion is:
Q \u003d um × s
If, as the surface is combustion, the particle is reduced, then such a form is called depressive (for example, ball, cube) if the surface increases - progressive (Checker with channels).
IN
Fig. 3. The burning of porch checkers
Combustion of condensed explosives
In the process of burning charges of condensed aircraft should be distinguished three main processes:
ignition from the ignition source from the charge of the charge;
layer burning;
inflammation of the charge with a side surface (in the absence of a booting side surface).
Theoretically described such a complex heterogeneous process as burning is quite difficult. But if you make a number of assumptions:
The entire reaction occurs at the maximum combustion temperature,
The substance first evaporates completely, and then reactions in pairs, etc., then the process is amenable to description.
According to theories of normal burning The rate of propagation of the chemical reaction zone is determined by a set of two processes:
● heat transfer from the chemical reaction zone to the heating zone due to thermal conductivity and
● mass transfer of substance from the warm-up zone to the chemical reaction zone due to diffusion.
With a joint solution of the equations of thermal conductivity and diffusion, the mass of mass combustion rate was obtained:
U. m. =
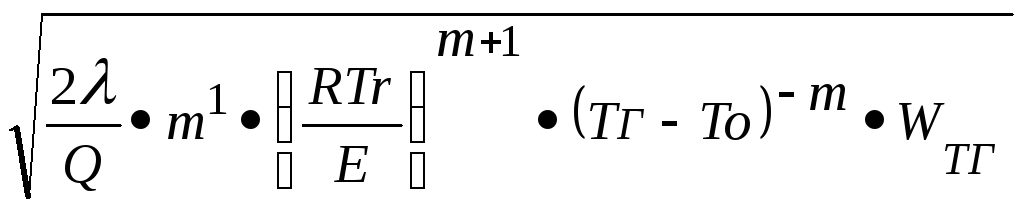
λ is the coefficient of thermal conductivity;
 -Provy effect of the oxidation reaction (from the unit of gas mass);
-Provy effect of the oxidation reaction (from the unit of gas mass);
m - the order of reaction;
T R - combustion temperature;
E - activation energy;
T 0 - initial temperature;
W T G is the rate of reaction at t of burning.
W T r \u003d ρ o · z · exp (-E / RT g).
FROM 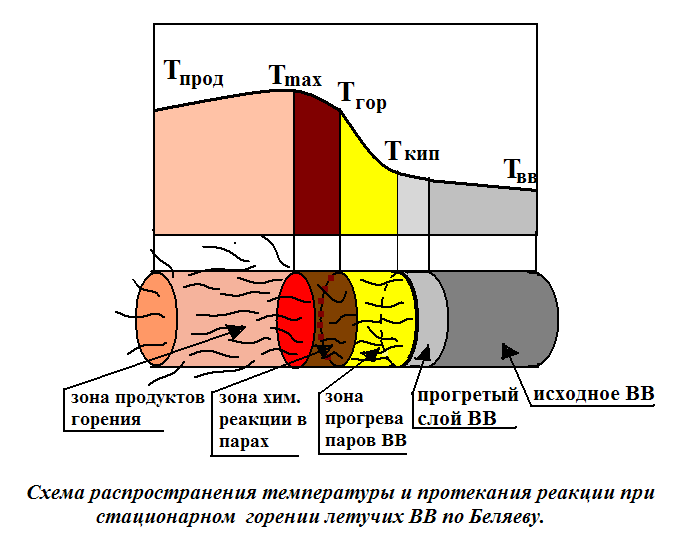 the temperature of the temperature and flow of the reaction in the stationary burning of the volatile explosions of Belyaev.
the temperature of the temperature and flow of the reaction in the stationary burning of the volatile explosions of Belyaev.
The dependence of the burning speed from various factors
Linear and mass combustion rate depends on:
geometric charge sizes (diameter);
external conditions (temperature and pressure);
dispersion of GS (particle size);
the presence of impurities.
For mix systems, additionally affect:
chemical nature of components, their thermophysical characteristics;
the ratio of components and dispersion of each of them.
The burning occurs in the result of the transfer of the adjacent heat layerhighlighted in the reacting layer. Simultaneously with the release of heat occur losses Its enable.
Burning proceeds stationaryonly in the event that the amount of heat given to the neighboring layer and the heat loss is equalized by the heat treatment due to the reaction.
If the total heat transfer becomes greater than the heat cride, the combustion is not possible.
With a decrease in the charge diameter, the amount of heat allocated per unit of time decreases in proportion to the square of the diameter. The heat transfer is also reduced, but slower - it is proportional to the surface of the heat sink, i.e. First degree of diameter. With some value of the diameter, the heat cride cannot compensate for heat loss and burning fades.
Critical burning diameter Call the minimum diameter at which there is still a burning. The critical diameter is not a constant value, it depends not only on the nature of the explosive, but also on the conditions of combustion, primarily on pressure, initial temperature, charge density and dispersion of crystals.
● The increase in temperature and pressure reduce the critical combustion diameter, increase the burning rate, reduce the loss into the environment.
The combustion of solid combustible substances in the initial stage of burning is called sunbathing. This is characterized by burning instability, relatively low temperatures in its zone, a small flame torch size and a small area of \u200b\u200bthe hearth.
The ambient temperature is raised slightly, only directly at the burning center.
The initial stage of fire (sunbathing) can be eliminated by primary fire extinguishing agents. If the fire is not immediately repayed, then the heat that is distinguished by burning will strengthen the process of the latter. At the same time, the size of the flame torch will increase and combustion will switch to a stable form. Simultaneously increases the ambient temperature and aggregate gee action of thermal energy emitted by the burning center. And the elimination of such a lighting requires a large number of primary means of fire extinguishing, water and foam jets.
With insufficient effectiveness of the extensive means of fire extinguishing or later, the combustion continues to develop, its zone increases on a significant area. This increases the temperature, there is a significant amount of thermal energy, convection air flows increase. Under the specified conditions, deformation and collapse of structures are possible.
To eliminate such a fire, you need a lot of strength and powerful tools.
The combustion rate of materials during a fire is different and depends on the conditions of combustion, the composition of the combustible substance and the transmission intensity of the last heat from the combustion zone.
There are two combustion speeds: weight and linear. Weight speed is called weight (int, kg. ) substances that burned per unit of time (in Min, Ch ). The linear rate of combustion of solid combustible substances is called the speed of propagation of fire (inm / min ) and the growth rate of the fire focus (in M. 2 / Min. ).
The combustion rate of solids is inconsistent and depends on the ratio of their surface to the volume, from humidity, air access and other factors.
Based on the data obtained in the study of a number of fire cases on river vessels, the linear rate of propagation of fire ranges from 0.05 to 2.5 m / min, and the growth rate of the fire focus is from 0.3 to 50.0 m 2 / min.
At the beginning of the emergence of a fire, about the first 2-3 minutes, there is an intensive increase in the area of \u200b\u200bits focus on passenger ships to 41-44 m 2 / Mia. This is explained by the fact that in this period a lot of time goes to collect the personnel of the vessel's crew and is not still being actively fighting fire. In the next 10 minutes, when stationary means of jodium and pennothession are introduced, the growth of the area of \u200b\u200bthe fire is slowed to approximately 6-7 m 2 / min.
The studies have established that the passenger vessel can be destroyed for 20-30 minutes if the organization of its extinguishing is imperfect.
The linear speed of the spread of fire determines the area of \u200b\u200bthe fire, and the degree of burnout in everything that can burn on this area is the duration of the fire.
The linear velocity of the liquid is the height of the layer of it (in mm, cm), burned per unit of time (in min, h).
Fire flame propagation rate when flammable combustible gases is from 0.35 to 1.0 m / s.
Speed \u200b\u200bburnout The amount of fuel, burning per unit of time from a unit of burning area. It characterizes the intensity of the combustion of the fluid during the fire. It must be known to determine the estimated duration of the fire in the tanks, the intensity of the heat dissipation and the temperature regime of the fire, etc.
The rate of burning in the fluid is inconsistent and depends on its initial temperature, the diameter of the reservoir, the level of the fluid in it, the content of non-combustible liquids, wind speeds and other factors.
In tanks with a diameter of up to 2 m, the rate of burning apart of liquids increases with its magnification. Almost it is the same in tanks, the diameter of which is more than 2 m.
The velocity of the fluid burnout, spilled on the surface, is approximately the same as in the tanks, if its thickness of its layer is significant
So, for example, the rate of burnout of oil is 25 cm / h , gasoline -40 cm / h, oil-20 cm / h.
With the flame burning of the petroleum product in the cargo tank, the fluid is heating.
The heating of the liquid from the upper to the lower layers occurs in the mass of heavy oils at a rate of 30 cm / h, and in the mass of light oil - from 40 to 130 cm / h.
Kerosene and diesel fuel during combustion are heated slowly, and the heated layer is not formed the same temperature.
Oil and fuel oil are heated intensively intensively, the layer temperature is almost always above 100 ° C. The temperature warms the oil layer can reach 300 ° C and heat the bottom layer of water in the tank.
The temperature of the heated layer of gasoline is usually below 100 ° C, therefore the bottom layer of water in the container does not heat up.
Warming fluid in tanks can lead to its boiling or emission. Under boiling it means the transition to a large number of small droplets of water in the petroleum product is understood. At the same time, a foam is formed on the surface of the liquid, which can be overflowed through the board of the tank. The emission is understood as the instantaneous transition of water at the bottom of the reservoir, in steam. In this case, the increased pressure is created, under the action of which the ejection of the burning fluid from the tank occurs.
The boiling of petroleum products in most cases is associated with the presence of water and less frequently water pillows at the bottom of the reservoir. All petroleum products containing water, which in the combustion process heats up above 100 ° C in the process of burning is capable of boiling
Oil and fuel oil are capable of pouring out only with a certain content of moisture in them: oil-3.3% and fuel oil - above 0.6%. "
Support can machine oil and heavy gasoline when you write a bottom layer of water.
Cooling by water jets of the reservoir walls and the periodic administration of a sprayed jet of water by one third or a quarter of the combustion surface prevent boiling and overflow the heated gasoline or oil from it.
If (the height of the free side exceeds the thickness of the heated layer more than 2 times, then when IB is administered to the combustion zone of the sprayed jet of water, boiling is observed, but the transfusion of the liquid from the container does not occur.
Dark oil products are capable of emission - oil containing 3.8% moisture, fuel oil containing up to 0.6% moisture.
The release of a burning fluid may occur if: under the layer it is water; The liquid during combustion is heated deep into; The temperature of the heated layer above the boiling point of water.
The release occurs at the moment when the oil product at the interface of the water - oil product is heated above 100 ° C (approximately 150-300 ° C). After the first emission, the layer of oil product heated to higher temperatures is in contact with water and a powerful emission occurs.
The release in height, range and area of \u200b\u200bthe lesion depends on the diameter of the tank. In the container with a diameter of 1.387 m, the mass of burning oil emitted outwards is from 51 to 145 kg at a height of 10 to 20 tank heights.
The duration of the ejection process from the container is from 3 to 60 seconds. The occurrence time is different, ranging from 2 to 5 h 30 min from the start of burning for various petroleum products at different tanks.
Usually the emission is accompanied by numerous tips of the petroleum product. The release of the total petroleum product in one tilt is a rare phenomenon and is observed with a small layer of the remaining petroleum product and its significant viscosity.
A characteristic sign of the start of the emission is the occurrence of vibrations of the walls of the capacity, accompanied by noise and increasing the size of the flame torch.
In the tanks of a larger diameter, the release is faster than in a small diameter tank. The magnitude of the water pillow layer on the emission does not affect.
The normal combustion rate of the gas and steam-air mixture is called the speed with which the boundary surface between the burnt and unburned gases relative to the unburned gas is located in the immediate vicinity of the combustion surface.
Speed \u200b\u200bfuel combustion rate
Linear combustion rate of solid fuel - the speed of moving the surface of burning deep charge - depends on the composition and charge manufacturing technology, charge temperature T 3,pressure in the chamber r,gas stream speeds along a burning surface V, fuel stretching, acceleration a \u003d ng,directed to the burning surface, as well as from other factors:
and \u003d u (t 3) f (p) fi (v) F 2 () F 3 (a).
Functions included in this dependence are assumed independent and are determined experimentally.
1. The dependence of the combustion rate on temperature is expressed in one of the following forms:
but) ![]() ;
;
b) ![]() ;
;
in) ![]() .
.
Constant D. 1 / B \u003d (1 ... 5) 10 3 1 / ° C, and large values \u200b\u200brefer to ballistic, and smaller - to mix solid fuels; accepted T n \u003d \u003d20 ° С.
2. The dependence of the gas burning rate is usually expressed in
One of the following forms:
but) u \u003d U;
b) u \u003d a + bp;
in) u \u003d. or u \u003d.
In the inner ballistics of RDTT, is used, as a rule, a power dependence and= u x P V,where v.\u003d 0.2 ... 0.8, and large v.belong to ballistic, and smaller - to mix solid fuels. For some fuel in a limited pressure range v.\u003d 0, can also be sections where v.< 0.
3. The burning speed depends on the speed of the gas stream along
burning surface starting with "threshold" flow rate
V. N. or other determining parameter. Dependencies are different
namely:
a) f.(v) \u003d L + k. V (V-V) at V V,
(for fuel JPN we have V \u003d 180 ... 200 m / s; k \u003d.0.0022 C / m) or f. \u003d 1 + k (n) with n; where for some ballistic fuels we have
; ![]() (and measured in cm / s, p. - in 10 MPa);
(and measured in cm / s, p. - in 10 MPa);
b) f.(v) \u003d L + k. v with V V,
where for ballistic fuel n
; V 140 ... 200m / s;
for example, p 0.4; to0,8;
d) for ![]() ,
,
where for ballistic fuels we have (S / F)100; k.0,003...0,004; S -the area of \u200b\u200bthe burning surface in the cross section with the coordinate x.:
1 at ![]()
d) 0,0125 ![]() for
for
where for ballistic fuel n we have (FGV, 1971, No.L) \u003d 0.04;
J. =1,6; J. n \u003d 5.6.
Factors k v, k, k, toand k.are not physical constants of fuel, but in the limited limits of a particular intraballastic calculation are made permanent. Fuel with low combustion rates are more susceptible to erosion burning than fuels with high speeds. Near V n at V< v n наблюдается уменьшение скорости горения (отрицательная эрозия, см. п.2.3.2).
4. Dependency of the burning rate from stretching deformation has
view f. 2 () = 1 + b;value b.- order of unity.
5. The burning of the combustion of solid fuel increases with the growth of UCN
rhenium nG,acting perpendicular to the burning surface; So,
for porch, we have (according to B. I. Goncharenko) that f. 3 (n.) =
equal to 1; 1.2; 1.4; 1.5 and 1.6 when p\u003d 0.7 10 3; 1 10 3; 4 10 3; 8 10 3 and 18 10 3, respectively.
For metallized mix solid fuels, in which the mass fraction of aluminum is z A 1, the relationship between f. 3 \u003d I. pit has appearance (FGV, 1978, No. 6):
![]() ,
,
where the pressure is measured in 10 Pa, the burning rate is in mm / s.
With very large accelerations (on the saturation site) for various fuels f. 3 () = 1,5 ...2,5 .
Increase andunder the action of acceleration depends on the size of the particles of aluminum contained in the mixed solid fuel. With the deviation of the acceleration vector from normal to surface effect pon the andfirst, it decreases approximately as a cosine of angle of inclination, and at angles 0 ... 70 0, the acceleration does not affect the burning rate.
The combustion rate of a non-measure composition of purified components does not change with an increase in overloads to 10 3 g.
6. The combustion of the combustion in the conditions of fast pressure differs from stationary value, and this change may be approximately described, for example, dependence
![]() ,
,
where \u003d 0.5 ... 2; but -fuel temperature coefficient.
It is possible to interrupt the burning of fuel at a fairly fast decline in pressure:
For ballistic fuels;
- u / D. - for blended (D -the diameter of the oxidant grain).
The impact of the combustion of various parts of solid fuel is also influenced by the design of the design, manufacturing and operating modes (storage) of RDTT.
Sustainable combustion of solid fuel is determined by the following heat sources:
1) total exothermic reactions flowing into a thin surface layer of fuel;
2) total exothermic processes occurring in the smoke mixture.
The heating of the fuel to the temperature necessary for sustainable burning is carried out mainly by the first source of thermal energy; At the same time, most of the fuel in the surface layer is dispersed.
With quasi-stationary burning of solid fuel at speeds andin the heated layer, the temperature distribution is established approximately described by exponential dependence (Fig. 2.1)
T (x) T 3+ (T S -) EXR ( -XU / A.),
where T S, T 3 -surface temperature of burning fuel and initial | Charge temperature.
For ballistic fuels there is a unambiguous dependence of the surface temperature T S.from the speed of burning and.For fuel H. T.equal to 600, 650, 690 and 720 k and\u003d 0.25; 0.5; 0.75 and 1 cm / s, respectively.
In total, the amount of heat is accumulated in the heated layer.
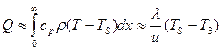 .
.
The main stock of this heat is imprisoned in a layer thickness \u003d a / andwhose warming time is order t. 4 = -A / and g(The thermal relaxation time for ballistic fuel is 60 and 4 ms at a pressure of 0.4 and 6.0 MPa, respectively). Based on this, it is possible to believe that to ignite the charge and sustainable development of the decomposition reaction, solid fuel is necessary to transmit a certain amount of heat to the surface layer. /andand heat the fuel surface to a temperature close to the value during a certain time, equal to approximately a / and 2.At the same time, the pressure in the RDTT should be greater than the magnitude necessary for sustainable burning.
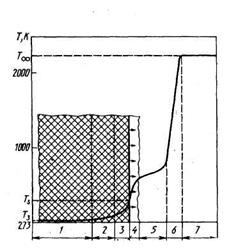 |
Fig. 2.1 Ballistic fuel combustion scheme:
T 3 -initial charge temperature; - temperature on the surface of the separation of solid and gas phases; 1 - initial fuel condition; 2 - zone of heating and primary decomposition of components; 3 - liquid layer; 4 - gasification zone; 5 - zone of preparation of a combustible mixture; 6 - burning zone; 7 - Products
combustion.
Increasing combustion rate with increasing pressure and charge temperature is due to the fact that under these conditions, the heating of the surface layer is accelerated. The growth rate of combustion at V\u003e V n is due to an increase in the effective thermal conductivity and diffusion coefficients in the developed turbulent flow. Under the action of overloading the agglomerates formed during burning, pressed against the surface and, being in size comparable from the thickness of the heated layer, increase locally heat transfer to fuel and lead the burning front. When tensile solid fuel, microcracks appear, available for burning, and the linear speed of moving the burning surface increases.
Specific parameters of the dependence of the combustion rate of each charge (or each batch of charges) of solid fuel from pressure and temperature (for example, and \u003d and (t 3) p v)defined by burning a cylindrical sample, reserved along the side surface, in a constant pressure device (Fig. 2.2). Error definition and= e / T.this device consists of measurement errors of several parameters:
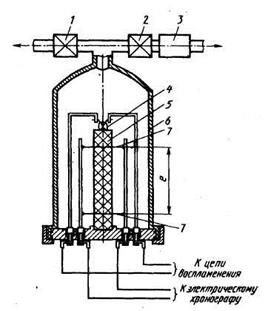 |
Fig. 2.2. Permanent pressure device for measuring the combustion rate of solid fuel:
1 - exhaust valve; 2 - inlet valve; 3 - gearbox in the pipeline from the balloon battery; 4Electric spiral ignition of solid fuel sample; 5 -Bered sample side surface; b - bomb of constant pressure; 7 - Wires, burning when passing the front of the combustion.
The radiation and the flow of gases in the constant pressure device differ from radiation and the flow of combustion products in the engine. Therefore, the value of the combustion rate measured in the constant pressure device is adjusted using an empirical coefficient. to I.\u003d 1 ... 1.1 for combustion conditions in the engine (with V< v n). Коэффициент k V,the effect of the gas flow rate on the combustion rate at V\u003e Vn is determined on special installations (for example, on the installation with the GG, similar to that shown in Fig. 5.42, where samples of solid fuels are placed instead of heat-shielding samples) or by burning charges in model RDTT .
In the device of constant pressure, burning of stretched samples is also carried out in order to obtain a value . The dependence of the combustion rate from acceleration is established when testing model RDTT, fixed on the centrifugal test bench or when testing rotating around the RDTT axis.
The addition of metal powders into solid fuels does not have a significant effect on the burning rate (in the absence of high accelerations aimed at the surface of the combustion), since the ignition and combustion of metals occurs in the gas flow. A distinctive feature of combustion of mixed metallized solid fuel is that it is a complex sequence of transforming parts of a metal (aluminum) - agglomeration (enlargement) at the reacting surface of the fuel, their ignition, removal in the gas phase, burning and movement in it. The oxidizing agent grains (ammonium perchlorate) is an order of magnitude and more exceed in size, the initial particles of aluminum contained in a combustible - binding, filling "pockets" between the grains. The burnout intensity is maximal in the area of \u200b\u200bborders with the latter. Therefore, when the wave of burning is passed, the fusion of metal particles accumulated in this pocket occurs, and these units per one - two orders of magnitude are larger than the initial particles. Under some conditions, the merger of aggregates from neighboring "pockets" can also occur, and the formation of several aggregates within one "pocket". From the subsequent movement and combustion of aluminum aggregates, coagulation and decay drops A1 / A1 2 O 3 depend on the loss of the specific impulse, the effects of multiphase flow of combustion products on thermal protection of RDTT and slag formation. As a result of the analysis of experimental data based on aluminum oxide particles in combustion products, the following formula was obtained:
where d. Measured VM; t. - in C; r - in MPa; d. - in μm; t.= L./ v; L -engine length.
COMBUSTION, Fiz.-Chem. The process, with a rum, the conversion of in-be is accompanied by intense excretion of energy and heat and p. Unlike and leaks with lower speeds and is not related to education. The combustion is based on Chem. The rival capable of proceeding with progressive self-esteem due to the accumulation of the released heat (thermal burning) or active PARS. Products (chain burning). Naib Walled thermal burning; The chain burning in its pure form is relatively rare, ch. arr. In the case of some gas phase P-thies at low.
Terms of thermal. Selfish m. b. Provided for all r-cues with sufficiently large thermal effects and. Naib Extensive class of district combustion-oxidation, for example. When burning Pri. , etc.; Oxidizers-oxygen ,. In combustion mode can occur: decomposition, dinitrichlilation, methylthitrate, etc.; Oxidis.-restore. p-rs, in reinstanders, elements with high affinity K (Ca, Al, Si, Mg, etc.); Synthesis of elements, halides, chalcogenides, refractory and.
The burning can begin spontaneously as a result or be initiated by ignition (see). At fixes. externally conditions (, T-ra, the size of the reactor, the parameters of heat and mass transfer, etc.) continuous combustion can flow in the stationary mode when the main. The characteristics of the process - the rate of R-│, the amount of heat released into a unit of time (heat generation capacity), T-ra and the composition of the products - do not change in time or in periodic. mode when these characteristics oscillate around their average values. Due to the strong nonlinear dependence of the rotation speed from T-ry, combustion is characterized by high sensitivity to external. Conditions: when they are insignificant. Change the slow rival can go into combustion mode or, on the contrary, developed burning may stop. This is a combustion of combustion determines the existence of several. Stationary modes under the same conditions (hysteresis effect).
The theory of burning.
With adiabatich. burning combustible mixture, i.e. In the absence between the reacting system and, m. b. The number of heat distinguished during burning, T-ra, K-paradium would have been achieved with full combustion (t. Naz. adiabatich. T-ra burning), and the composition of the products, if the composition of the initial mixture and thermodynamic is known. F-nations of the source mixture and products. If the composition of the products are known in advance, t m. B. Calculated from the condition of equality. The energy of the system (with the post. volume) or its (with the post.) In the initial and finite states with the help of the relation: T g \u003d T 0 + QR / C, where t 0-initial T-ra mixture, with-average in the interval T -R from T 0 to T T G Ud. The initial mixture (taking into account its change with possible), (q g-form-mixture at T-RA with the relative content of A 0 in the mixture of the components fully consumed in the r application (eg, fuel), q r \u003d Q * A 0 where the Q-thermal effect of combustion is the value of TP with the post, the volume is greater than with the post., Since in the latter case, part is internal. The energy of the system is spent on the work of the expansion. In practice, adiabatich conditions are ensured in those Cases when the rival has time to end before it becomes essential between the reaction. volume and, for example, in the combustion chambers of large jet engines, in large reactors, with rapidly propagating burning waves.
Thermodynamic. The calculation provides only partial information about the process - the equilibrium composition and T-RP products. A complete description of the combustion, which also includes the determination of the speed of the process and critic. Conditions in the presence of heat and C, can be carried out only within the framework of Macroneetich. The approach considering chemicals. R production in relationship with energy and in-va (see). In the case of a pre-mixed mixture of combustible and combustion, combustion can occur in the entire space occupied by a combustible mixture (volumetric combustion), or in a relatively narrow layer separating the original mixture and products and propagating the combustible mixture in the form of T. Naz. Waves of burning. In unsecured systems, diffusion combustion is possible, with a rum, the rival is localized in a relatively thin zone separating fuel from, and is determined by the speed in this zone.
Volumetric burningit happens, for example, in the thermal insoles. The reactor is ideal (see), in K-ry comes at T-REA 0 The initial mixture with refers. the content of fuel and 0; Upon other T-re g, the reactor leaves the mixture with another. The content of fuel a. With the full flow rate G through the reactor, the conditions for the balance of the mixture and the content of combustible in the stationary combustion mode can be recorded by urms:
where W (A, T) is the rod of the combustion, the reactor. Using the expression for thermodynamic. T-ig T G, can be obtained from (1): a \u003d a 0 (T g - T) / (T G - T 0) and write (2) in the form:
where q - (t) \u003d Gc (T - T 0) - the heat of the heat removal from the reactor with the combustion products, Q + (T) \u003d QW (A, T) V-speed of heat isolating during the r application. For the R№ and-th order with:
(k 0 -PedExponenz. Multiplier in Arrhenius URS). On the Q - T diagram (Fig. 1), the dependence Q - (T) is expressed by a straight line, the angle of inclination to-point is the greater, the more flow rate through the reactor; Q + (T) is expressed by a curve with a sharp maximum near the city of the rising branch of this curve due to the rapid increase in the speed of the R-JEC with T-Roy (in the expression for W, the contribution gives exponentials. Multiplier); with it means. The burnout of the combustible mixture is very diluted with products, the prevailing effect on the rate of the R-α begins to give a multiplier and n and the r-α slows down sharply. Since the combustion p knee is characterized by large values \u200b\u200bof E, the maximum on the curve q + (t) is expressed very sharply and is strongly shifted to T g, i.e. Naib The mixture is quickly reacting, highly heated with high heat, although significantly diluted with products. When Costs G Balance conditions (1) and (2), to-ryn correspond to the intersection points Q + (T) and Q - (T), can be performed with respect. T-rah. Accordingly, the rival may proceed in different ways: in low-temperature mode without progressive selfishness, with insignificant. self-dissemination (t t 0) and burning of fuel (AA 0) (point A in Fig. 1 at the flow rate G T) or in combustion mode at high T-rah (t t y) and large degrees of burnout (A0) (point Fig. 1 at consumption G 3). The transitions between these two modes-ignition of the mixture and its extinction - occur jumps like criticism. Costs G B and G P ass., and always g b< G П. При промежут. расходах G B
< G < G П возможен также неустойчивый режим протекания
р-ции при нек-рой промежут. т-ре (точка В на рис. 1 при расходе
G 2), когда любое малое случайное возмущение расхода приводит
р-цию в один из устойчивых режимов (А" или С). Гистерезисный
эффект, свойственный горению, заключается в том, что при любом расходе G в интервале
от G B до G П м. б. реализованы оба устойчивых режима
- высокотемпературный (собственно горение) и низкотемпературный, в зависимости
от того, достигнуто ли данное значение G увеличением расхода со стороны
значений, меньших G B , или уменьшением его со стороны значений,
больших G П.
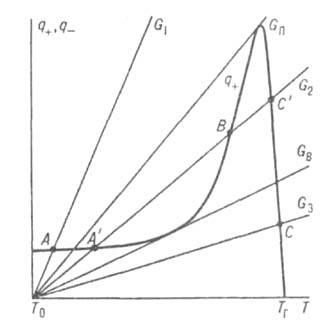
Fig. 1. The dependence of the heat generation rates of Q + and the heat sink q - from the T-FT of the reactive system at the time. flow values \u200b\u200bthrough reactor C (explanation in text); T 0 -T-ra. T G - Adiabatich. T-ra burning.
Critic. and hysteresis phenomena characteristic of combustion occur not only when a consumption is changed, but also by changing other external. Conditions (T 0, V, etc.).
Waves burningconducted by a characteristic bond burning-ability to spread in space occupied by a combustible mixture. Began in one layer of a combustible mixture filling K.L. volume, eg The pipe, the combustion rod is initiated in the adjacent layers due to their heating with hot products or due to the reacting layer. As a result, the combustion front spreading along the pipe is arising, in front of which there is a combustible mixture at the initial T-REA 0, behind it-products of combustion at T-RE T P. In the absence of heat loss through the walls of the pipe T n \u003d t G. in Stationary combustion mode All points of the flat wave front are moved at the same speed M, constant in time. The velocity W, T soluble and the combustible components are unevenly distributed in the combustion front, forming along the coordinate of the front of the front of the three zones (Fig. 2). In T. Naz. Zone 1 T-ra takes values \u200b\u200bin a narrow interval ![]() Near T r, and the rate of r-│ maximum. In the warming zone, 2 speeds of ration and heat dissipation are significantly less, the OSN. The role in the thermal balance of the mixture plays a heat flux from the zone of the Rocation. Under the influence of this flux, the combustible mixture heats up so quickly to high T - R, which components do not have time to react. In Zone 3, the molecular components of the combustible mixture and product products are carried out. As a result of fuel in the district zone, it is very reduced and the mixture is enriched with combustion products. The width values \u200b\u200bof the warm-up zone L T and zone L D are determined by the COEF. The temperature of the mixture of the mixture and products D is acc.:. The width of the Zone zone L p for a simple one-step solution is many times less than L T: L P / L T ~ RT T 2 / E (T G - T 0). In cases of the R-│ with a complex mechanism (for example, with strong braking of the R production by the product) L R m. B. comparable and even exceed L.
Near T r, and the rate of r-│ maximum. In the warming zone, 2 speeds of ration and heat dissipation are significantly less, the OSN. The role in the thermal balance of the mixture plays a heat flux from the zone of the Rocation. Under the influence of this flux, the combustible mixture heats up so quickly to high T - R, which components do not have time to react. In Zone 3, the molecular components of the combustible mixture and product products are carried out. As a result of fuel in the district zone, it is very reduced and the mixture is enriched with combustion products. The width values \u200b\u200bof the warm-up zone L T and zone L D are determined by the COEF. The temperature of the mixture of the mixture and products D is acc.:. The width of the Zone zone L p for a simple one-step solution is many times less than L T: L P / L T ~ RT T 2 / E (T G - T 0). In cases of the R-│ with a complex mechanism (for example, with strong braking of the R production by the product) L R m. B. comparable and even exceed L. 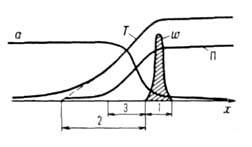
In accordance with the current values \u200b\u200bof T and A is distributed at the combustion front and the total mixture of H (Fig. 3). With heated layers enriched with a combustible mixture have an excess compared to H 0 of the initial mixture; It has been printed and strongly diluted with food mixture has a flaw. Excess in front of the combustion-cause of the instability of stationary burning waves and the occurrence of oscillatory modes of their distribution. 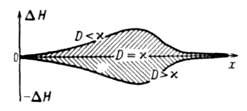
Fig. 3. Changing the reacting system along the coordinates of the propagation of the combustion front; and D-coefficients of the temperature of the mixture and products, respectively.
In case of complex rnesses occurring in a multi-stage mechanism, the structure of the combustion front may turn out to be more complex than in the case of a simple (single-dine) R-│. Depending on the ratio between kinetich. Split. Stages of complex P production These stages can either flow in one zone (merge mode), or can be spatially separated and completed. Between both thermal and diffusion streams (control mode), or there will be no mutual influence (separation mode). The speed of spreading the front of burning with several. The zones of the Rocation are usually determined by K.L. One of them (t. Naz. leading zone).
Number of fuel, burning on a unity of the front of the front of the combustion per unit of time, called. Most combustion rate t. It is determined by the expression: 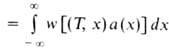 where the density of the initial mixture.
where the density of the initial mixture.
The calculation of the combustion velocities M and linear U-is associated with finding the distribution of T - and all components of the mixture at the burning front and requires a joint solution of differentials. Heat and mass transfer urins in a reacting medium. According to Zeldovich-Frank-Kamenetsky, for simple (single-dine) Р-
where-thermal conductivity of the mixture, \u003d Rt 2 g / e (t g - t 0); Values \u200b\u200band W correspond to T-RE T G, the density of the mixture and efficient flammable in the zone of the R-│. From this F-lia it follows that from all physical chemical. SV-in combustible mixture and characteristics of heat and mass-rhenosa is NaB. The influence of T solisses T solitions, since the dependence of T from T g corresponds to the exponent. law, i.e.
![]()
In real conditions, the spread of the combustion front is always accompanied by heat loss in external. Wednesday (radiation,), which leads to a decrease in t-ry and burning rate compared to their adiabatich. values. If the ratio of the intensity of heat loss to the power of heat dissipation exceeds some critical. The value, the self-proliferation of the ration on the combustible mixture becomes impossible. The cutting of burning with increasing heat loss is carried out by a jump: immediately before swelling, the burning speed is different from zero and even m. B. Close to the speed of adiabatich. burning. With the breakdown of burning due to heat loss, the concepts are associated. The limits of burning. So, if the content in the combustible mixture becomes less stoichiometric, strongly decreases the rates of ration and heat dissipation. With unchanged heat transfer, this leads to an increase in the ratio of the intensity of heat loss to the power of heat dissipation. With some fuel, this attitude reaches criticism. values \u200b\u200bbelow the mixture becomes non-combustible in these conditions; The corresponding fuel is called. Concentration limit of burning. Similarly, the limits of burning on the initial T-RE, the diameter of the pipe, etc.
Diffusion burningtakes place in conditions when fuel and diffuse in the zone of the Rocation from the opposite sides; Such, for example, burning candles, wicking. If, at the same time, k r-│ burning is much less, they have time to move and the ration proceeds in ordinary kinetich. mode (relatively low-temperature). For