What trace elements are part of a bone skeleton of a person? The structure of bone tissue. The bone tissue includes, as known, bone cells and an intercellular substance, which consists of the main structureless substance and the decorated part in the form of
Biochemistry of bone tissue
Bone tissue - a special look connective tissue. Cellular elements of bone tissue are osteoblasts, osteocytes, osteoclasts.
Osteoblast Enough a large number of Glycogen, glucose. ATP synthesis is 60% connected with glycolysis reactions. The CTC reactions proceed in the cells, and citrate synthesis has the greatest activity. The synthesized citrate is used in the further binding of CA 2+ required in the mineralization processes. Since the function of Osteoblast is the creation of an organic intercellular matrix, these cells contain a large amount of RNA required for protein synthesis. Osteoblasts are synthesized and released into the extracellular space of glyceluphospholipids that bind calcium and participate in mineralization processes. Osteoblasts are synthesized and isolated in the intercellular substance of collagen fibrils, proteoglycans and glycosaminoglycans and ensure the continuous growth of hydroxyapatite crystals. As aging, the Osteoblasts turn into osteocytes.
Osteocyte Mature abnormal cell of bone tissue, generating components of the intercellular substance. Osteocytes in contact with each other through the proof.
Osteoclasts- Frame from macrophages, contain many lysosomes and mitochondria. They carry out a continuous managed process of reconstruction and renewal of bone tissue.
Chemical composition bone tissue
The intercellular organic compact bone matrix is \u200b\u200babout 20%, inorganic substances 70%, water 10%.
The intercellular substance consists of a basic substance (consisting of extracellular liquid, glycoproteins, proteoglycans), collagen fibers (90-95%), mineralsrepresented by crystals, mainly hydroxyapatite Ca 10 (PO 4) 6 (OH) 2. In addition, the ions of Mg 2+, Na +, K +, SO 4 2-, NSO 3-, hydroxyl and others were detected in the bone.
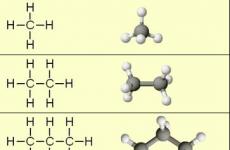
The main proteins of the intercellular matrix are collagen type I typewhich make up about 90% of the organic bone matrix. Type I collagen contains 33% glycine, 21% of proline and hydroxyproline, 1% hydroxylizin and a small amount of carbohydrates. Located in bones, dentin, tooth pulp, cement, periodontal fibers. This type of collagen fibers participates in mineralization processes. The primary collagen structure is represented by α-chains consisting of 1000 amino acid residues. Three alpha chains are twisted with each other and form tripocolelagen. Forming fibrils, the tropocolalagen molecules are located stepped, shifting relative to each other by one quarter of the length, which gives fibrils characteristic allocations. (Fig. 1)
Fig. 1. Collagen structures.
Hydrogen bonds arise between alpha chains of tropocolegated, in the formation of hydroxyproline, hydroxylyzine and glycosylated hydroxylyzine. Ascorbic acid is involved in the reactions of hydroxylation of proline and lysine. In the future, with the help of Lysyloxidase, the enzyme containing Cu 2+ is formed aldehyde derivatives of lysine and 5-hydroxylizin, which contribute to the formation of intermolecular covalent bonds between collagen fibrils. The formation of intermolecular bonds affects the strength of collagen fibrils.
Therefore, the disadvantage of ascorbic acid (ration), Cu 2+ ions, genetic defects, autoimmune states lead to a violation of collagen synthesis. Clinical manifestations will be in the form of changes from the dental system: bleeding of gums, mobility and loss of teeth, multiple caries.
Bone tissue contains about 10% non-flaked proteins. They are presented: 10% proteoglycans, 15% bone sialoprotein, 15% osteurectin, 10% α 2 Hsglycoprotein, 3% albumin serum, 15% osteokalcin, 32% other proteins. These proteins are synthesized with osteoblasts and are able to bind phosphates or calcium.
The intercellular organic compact bone matrix is \u200b\u200babout 20%, inorganic substances - 70% and water - 10%. The sponge bones are dominated by organic components, which make up more than 50%, the share of inorganic compounds accounts for 33-40%. The amount of water is approximately the same as in the compact bone.
Organic bone fabric matrix.Approximately 95% of the organic matrix falls on type I collagen. This type Collagen also includes tendons and skin, but the collagen of bone tissue has some features. It has several more oxyprolin, as well as free amino groups of lysine and oxyllisan residues. This causes the presence of more transverse bonds in collagen fibers and their greater strength. Compared with the collagen of other tissues, bone collagen is characterized by an increased phosphate content, which is mainly associated with serine residues.
The proteins of noncallagenic nature are represented by glycoproteins, protein components of proteoglycans. Take part in the growth and development of bones, the process of mineralization, water-salt metabolism. Albumin participate in the transport of hormones and other substances from the blood.
The prevailing protein of non-core nature is ostocalcin. It is present only in the bones and teeth. This is a small (49 amino acid residues) of a protein, also called bone glutamic protein or GLA protein. Three residues were found in the osteocalcin molecule
γ-carboxyglutamic acid. Due to these residues, it is able to bind calcium. For the synthesis of osteokalcin, vitamin K is required (Fig. 34).
Fig. 34. Post-translation modification of osteocalcin
The organic matrix of bone tissue includes glycosaminoglycans, the main representative of which is chondroitin-4-sulfate. Chondroitin-6-sulfate, keratan sulfate and hyaluronic acid are contained in small quantities. Ocanification is accompanied by a change in glycosaminoglycanov: sulfated compounds are inferior to non-malfated. Glycosaminoglycans are involved in the binding of collagen with calcium, regulation of water and salt metabolism.
Citrate is necessary for bone mineralization. It forms complex compounds with calcium and phosphorus salts, providing the possibility of increasing their concentration into tissue to such a level in which crystallization and mineralization can begin. It will also take part in the regulation of the level of calcium in the blood. In addition to citrate, the bone tissue found succinate, fumarate, malate, lactate and other organic acids.
The bone matrix contains a small amount of lipids. Lipids play a significant role in the formation of cores of crystallization during bone mineralization.
Osteoblasts are rich in RNA. The high content of RNA in bone cells reflects their activity and constant biosynthetic function.
The inorganic composition of bone tissue.
At an early age in bone tissue, amorphous calcium phosphate Ca 3 (PO 4) 2 prevails. In mature bone, the predominant becomes the crystalline hydroxyapatite Ca 10 (PO 4) 6 (OH) 2 (Fig. 35). Its crystals have the form of plates or chopsticks. Usually, the amorphous calcium phosphate is considered as a labile reserve of Ca 2+ ions and phosphate.
The composition of the mineral phase of bones includes sodium, magnesium ions, potassium, chlorine, etc. In the crystalline hydroxyapatite grid, the Ca 2+ ions can be replaced by other bivalent cations, while anions other than phosphate and hydroxyl are or adsorbed on the surface of crystals, or dissolve in Hydrate shell crystal lattice.

Fig. 35. The structure of the crystal of hydroxyapatitis
Metabolism of bone tissueit is characterized by two opposing processes: the formation of new bone tissue with osteoblasts and resorption (degradation) of old osteoclasts. Normally, the number of newly formed tissue is equivalent to destroyed. The bone tissue of the human skeleton is almost completely rebuilt for 10 years.
Bone fabric formation
On the 1 stageosteoblasts are synthesized first proteoglycans and glycosaminoglycans, forming matrix, and then produce bone collagen fibrils that are distributed in the matrix. Bone collagen is a matrix for the mineralization process. A prerequisite for the mineralization process is the submission of calcium and phosphorus ions. The formation of crystals of the mineral cities are launched
Sa-binding proteins on the collagen matrix. Osteocalcin is firmly associated with hydroxyapatite and participates in the regulation of crystal growth due to the binding of Ca 2+ in the bones. Electronic microscopic studies have shown that the formation of a mineral crystal lattice begins in areas located in regular intervals between collagen fibrils. The resulting crystals in the collagen zone are then becoming cores of mineralization, where hydroxyapatite is deposited in space between collagen fibers.
On the 2 stage In the mineralization zone, with the participation of lysosomal proteinases, proteoglycanov degradation occurs; Oxidative processes are enhanced, glycogen decomposes, the required amount of ATP is synthesized. In addition, osteoblasts increase the amount of citrate required for the synthesis of calcium amorphous phosphate.
As the bone tissue is mineralized, the hydroxyapatite crystals displaces not only proteoglycans, but also water. Dense, fully mineralized bone is practically dehydrated.
Enzyme alkaline phosphatase takes part in mineralization. One of the mechanisms for its action is the local increase in the concentration of phosphorus ions to the saturation point, followed by the process of fixing calcium-phosphoric salts on the organic matrix of the bone. When the bone tissue is restored after fractures, the content of alkaline phosphatase in the bone corn increases dramatically. With violation of costh formation, there is a decrease in the content and activity of alkaline phosphatase in the bones, plasma and in other tissues.
Inorganic pyrophosphate is an inorganic pyrophosphate. A number of researchers believe that the process of mineralization of collagen in the skin, tendons, vascular walls prevents the constant presence in these tissues of proteoglycans.
Simulation and remodeling processes provide constant bone updates, as well as the modification of their shape and structure. Modeling (new bone formation) takes place mainly in childhood. Remodeling is the dominant process in the skeleton of adults; In this case, only replacement separate plot Old bone. Thus, in physiological and pathological conditions, not only education, but also resorption of bone tissue occurs.
Catabolism of bone tissue
Almost at the same time there is a "dissipation" of both mineral and organic bone fabric structures. In osteolysis, the production of organic acids is enhanced, which leads to a pH shift in the acidic side. This contributes to the dissolution of mineral salts and their removal.
Resorption of an organic matrix occurs under the action of lysosomal acid hydrolylase, the spectrum of which in bone tissue is quite wide. They are involved in the intracellular digestion of fragments of resorbable structures.
With all diseases of the skeleton, violations of the bone remodeling processes occur, which is accompanied by the occurrence of deviations in the level of biochemical markers.
There are common new bone fabric formation markers, such as bone-specific alkaline phosphatase, plasma osteokalcin, punctured I, plasma peptides. To biochemical bones resorption markers Calcium in urine and hydroxyproline, urine pyridinoline and deoxypyridinoline, which are transimatic transimatic fibers of collagen specific for cartilage and bones.
Factorsaffecting the metabolism of bone tissue are hormones, enzymes and vitamins.
Mineral bone tissue components are practically in a state of chemical equilibrium with calcium ions and serum phosphate. In regulation of the receipt, deposit and allocation of calcium and phosphate, pararathgarmon and calcitonin play an important role.
The action of the parathgamon leads to an increase in the number of osteoclasts and their metabolic activity. Osteoclasts contribute to the accelerated dissolution of mineral compounds contained in bones. Thus, the activation of cellular systems involved in bone resorption occurs.
Paranthgump also increases the reabsorption of Ca 2+ ions in the renal tubules. The total effect is manifested in increasing the level of calcium in serum.
The calcitonin action consists in reducing the concentration of Ca 2+ ions due to its deposition in bone tissue. It activates the enzyme system of Osteoblasts, increases the mineralization of the bone and reduces the number of osteoclasts in the zone of action, i.e. inhibits the process of bone resorption. All this increases the rate of bone formation.
Vitamin D is involved in the biosynthesis of CA 2+-binding proteins, stimulates the absorption of the calibulation in the intestine, increases the reabsorption of calcium, phosphorus, sodium, citrate, amino acids in the kidneys. With a lack of vitamin D, these processes are broken. Taking for a long time of excess amounts of vitamin D leads to the demineralization of bones and an increase in the concentration of calcium in the blood.
Corticosteroids increase the synthesis and secretion of the parathgamon, increase the demoormalization of the bone; Sex hormones accelerate maturation and reduce the bone growth period; Tyroxin enhances the growth and differentiation of the tissue.
The effect of vitamin C on the metabolism of bone tissue is due primarily to the influence on the process of collagen biosynthesis. Ascorbic acid is a cofactor of the shed and lysilhydroxylase and is necessary for the implementation of the reaction of the hydroxylation of Proline and lysine. The lack of vitamin C leads to changes in the synthesis of glycosaminoglycans: the content of hyaluronic acid in bone tissue increases several times, while chondroitin sulfate biosynthesis slows down.
With a lack of vitamin A, there is a change in the shape of the bones, a violation of mineralization, a height delay. Think that this fact due to disruption of chondroitin sulfate synthesis. High doses of vitamin A lead to excessive bone resorption.
With the lack of vitamins of the group, the bone growth slows down, which is associated with a violation of protein and energy exchange.
Dental fabric features
The main part of the tooth is dentine. Speaking of gums of the tooth, crown, covered enamel, and the root of the tooth is covered dental cement. Cement, dentin and enamel are built like bone tissue. The protein matrix of these fabrics consists mainly of collagen and proteoglycans. The content of organic components in cement is about 13%, in dentine - 20%, in enamel - only 1-2%. The high content of mineral substances (enamel is 95%, dentin - 70%, cement - 50%) determines the high hardness of the toothbrush. The most important mineral component is hydroxyapatite [Ca 3 PO 4) 2] 3 sa (OH) 2. It also contains carbonate apatite, chloroapatitis and strontium apatite.
Enamel covering tooth, semi-permeable. It participates in the exchange of ions and molecules with saliva. On the permeability of the enamel affects the pH of saliva, as well as a number of chemical factors.
In an acidic medium, the tooth fabric is attacked and lost hardness. Such a common disease as cariesis caused by microorganisms living on the surface of the teeth and the organic acids that wash out anaerobic glycolysis as an anaerobic glycolyising as an anaerobic glycoxy product.
Control questions
1. Name the main organic components of bone tissue.
2. What inorganic compounds are included in bone tissue?
3. What is the difference in biochemical processes occurring in osteoclasts and osteoblasts?
4. Describe the bone formation process.
5. What factors affect the formation of bone tissue and its metabolism?
6. What substances can be biochemical markers processes occurring in bone tissue?
7. What are the features of the biochemical composition of the toothbrush?
Literature
1. Birch, etc. Biological chemistry. / T.T. Berezov, B.F. Korowkin. - M.: OJSC "Publishing House" Medicine ", 2007. - 704 p.
2. Biochemistry. / Ed. E.S. Severin. - M.: Goeotar Media, 2014. -
768 p.
3. Biological chemistry with exercises and tasks. / Ed. E.S. Severin. - M.: Goeotar Media, 2013. - 624 p.
4. Zubairov, D.M. Guide to laboratory classes on biological chemistry. / D.M. Zubairov, V.N. Timerbaev, V.S. Davydov. - M.: Goeotar Media, 2005. - 392 p.
5. Swedov, V.N. Biochemistry. / Hard. Swedov. - M.: Yurait, 2014. - 640 p.
6. Nikolaev, A.Ya. Biological chemistry. / AND I. Nikolaev. - M.: Medical Information Agency, 2004. - 566 p.
7. Kushmanova, OB Guide to laboratory classes on biological chemistry. / O.B. Kushmanova, G.I. Ivchenko. - M. - 1983.
8. Lyninger, A. Fundamentals of biochemistry / A. Leninger. - M., "Peace". - 1985.
9. Marry, R. Biochemistry of man. / R. Marry, D. Grenner, P. Maes, V. Rozwell. - T. 1. - M.: Mir, 1993. - 384 p.
10. Marry, R. Biochemistry of man. / R. Marry, D. Grenner, P. Maes, V. Rozwell. - T. 2. - M.: Mir, 1993. - 415 p.
SA 10 hydroxylapatite crystals (PO 4) 6 (OH) 2 are part of the mineral phase of bone tissue, have a form of plates or chopsticks. Another part is represented by amorphous calcium phosphate Ca 3 (PO 4) 2. Calcium amorphous phosphate prevails at an early age, crystalline hydroxyapatite becomes the predominant in mature bones. Usually, the amorphous calcium phosphate is considered as a labile reserve of Ca 2+ ions and phosphate.
The composition of the mineral phase of bones includes sodium, magnesium ions, potassium, chlorine, etc. In the crystalline hydroxyapatite grid, the Ca 2+ ions can be replaced by other bivalent cations, while anions other than phosphate and hydroxyl are or adsorbed on the surface of crystals, or dissolve in Hydrate shell crystal lattice.
Organic bone fabric matrix.Approximately 95% of the organic matrix falls on collagen. Collagen is the main factor determining the mechanical properties of the bone. Collagen bone matrix fibrils are formed by type 1. This type of collagen is also included in the tendons and skin, but the collagen of bone tissue has some features. In bone collagen, there are several more oxyprolin than in the collagen of tendons and leather. For bone collagen, a large content of free amino groups of lysine and oxilizine residues is characterized. Another feature of bone collagen is an increased phosphate content in comparison with the collagen of other tissues. Most of this phosphate is associated with serine residues.
In a dry demineralized bone matrix, about 17% of non-core proteins are contained, among which protein components of proteoglycans are also located. In general, the number of proteoglycans in the formed dense bone is small.
The organic matrix of bone tissue includes glycosaminoglycans, the main representative of which is chondroitin-4-sulfate. Chondroitin-6-sulfate, keratan sulfate and hyaluronic acid are contained in small quantities.
Ocanification is accompanied by a change in glycosaminoglycanov: sulfated compounds are inferior to non-malfated. The bone matrix contains lipids, which are the direct component of bone tissue, and are not an impurity as a result not enough complete removal rich bone marrow lipids. Lipids can play a significant role in the formation of cores of crystallization during bone mineralization.
Osteoblasts are rich in RNA. The high content of RNA in bone cells reflects their activity and constant biosynthetic function. A feature of the bone matrix is \u200b\u200ba high citrate concentration: about 90% of its total amount in the body falls on the share of bone tissue. Citrate is necessary for bone mineralization. It forms complex compounds with calcium and phosphorus salts, providing the possibility of increasing their concentration into tissue to such a level in which crystallization and mineralization can begin. In addition to citrate, the bone tissue found succinate, fumarate, malate, lactate and other organic acids.
Bone formation. The formation of the intercellular substance and the mineralization of bone tissue is the result of the activities of osteoblasts, which, as bone tissue formation, is immutted in the intercellular substance and become osteocytes. The bone tissue serves as the main calcium depot in the body and is actively involved in calcium exchange. Calcium release is achieved by destruction (resorption) of bone tissue, and its binding is by forming bone tissue. With this, the process of constant restructuring of bone tissue continuing throughout the body's life is connected. In this case, there are changes in the shape of the bone according to changing mechanical loads. A human skeleton bone tissue is almost completely rebuilt every 10 years.
The health process is possible only in the presence of strictly oriented collagen fibers. The structural feature of the collagen fiber is that the tropocolelagen molecules located in a row are not connected to the end to the end. Between the end of the same molecule and the beginning of the next interval. It is likely that the gaps along the row of tropocoland molecules are the initial centers of mineral deposits. component parts bone tissue. The resulting crystals in the collagen zone are then becoming cores of mineralization, where hydroxyapatite is deposited in space between collagen fibers.
In the formation of bones in the calcification zone with the participation of lysosomal proteinases, proteoglycans are degradation. As the bone tissue is mineralized, the hydroxyapatite crystals seem to be displaced not only proteoglycans, but also water. Dense, fully mineralized bone is practically dehydrated. Under these conditions, collagen is approximately 20% of the mass and 40% of bone tissue, the rest falls on the share of mineral components.
Not all collagen-containing fabrics in the body are subject to ossification.
Apparently, there are specific calcification inhibitors. A number of researchers believe that the process of mineralization of collagen in the skin, tendons, vascular walls prevents the constant presence in these tissues of proteoglycans. There is also the opinion that inorganic pyrophosphate may be an inhibitor of calcification. In the mineralization of tissues, the inhibitory effect of pyrophosphate is removed by pyrophosphatase, which, in particular, is detected in bone tissue. In general, biochemical mechanisms of bone mineralization require further research.
The problem of catabolism of the bone matrix is \u200b\u200balso the problem. Both in physiological and in pathological conditions there is resorption of bone tissue, at which there is practically at the same time "dissipation" of both mineral and organic bone tissue structures. In the removal of mineral salts, a certain role belongs to the osteolysis of products of organic acids, including lactate. It is known that the shear of the pH of the fabric in the acidic side contributes to the dissolution of minerals and thereby removing them.
Resorption of an organic matrix requires the availability and action of the corresponding enzymes. These include lysosomal acid hydrolynes, the spectrum of which in bone tissue is quite wide. They are involved in the intracellular digestion of fragments of resorbable structures.
Therefore, in order to occur intracellular hydrolysis, the structure of organic matrix is \u200b\u200bnecessary to pre-expose, as a result of which fragments of polymers would have been formed. Thus, the resorption of collagen fibers requires the prior effect of collagenolytic enzymes.
The factors affecting the metabolism of bone tissue should first include hormones, enzymes and vitamins.
Mineral bone tissue components are practically in a state of chemical equilibrium with calcium ions and serum phosphate. Admission, deposit and isolating calcium and phosphate are regulated by a very complex system in which, among other factors, an important role belongs to parathgormon and calcitonine. With a decrease in the concentration of Ca 2 ions in the serum, the secretion of the parathgamon increases. Directly influenced by this hormone in bone tissue, cellular systems are activated involved in bone resorption (increasing the number of osteoclasts and their metabolic activity), i.e. osteoclasts contribute to increased dissolution of mineral compounds contained in bones. Paranthgump also increases the reabsorption of Ca 2+ ions in the renal tubules. The total effect is manifested in increasing the level of calcium in serum. With an increase in the content of Ca 2+ ions in serum, the calcitonin hormone is secreted, the action of which consists in a decrease in the concentration of Ca 2+ ions due to its deposit in bone tissue. It increases bone mineralization and reduces the number of osteoclasts in the zone of action, i.e. inhibits the bone resorption process. All this increases the rate of bone formation.
In the regulation of the content of ions Ca 2+, an important role belongs to vitamin D, which is involved in the biosynthesis of Ca 2+-binding proteins. These proteins are necessary for suction of ions Ca 2+ in the intestine, reabsorption them in the kidneys and mobilization of calcium from bones. Admission to the body of optimal quantities of vitamin D is prerequisite For the normal flow of bone calcification processes. If vitamin D deficiency, these processes are broken. Taking for a long time of excess amounts of vitamin D leads to bone demineralization. The cessation of bone growth is an early manifestation of vitamin A. insufficiency believe that this fact is due to a disruption of chondroitin sulfate synthesis. With the introduction of high doses of vitamin A, exceeding physiological need and causing the development of hypervitaminos A, observed bone resorption, which can lead to fractures.
Vitamin C is necessary for the normal development of bone tissue. The action of vitamin C is not metabolism of bone tissue is due primarily to the influence on the process of collagen biosynthesis. Ascorbic acid is necessary for the reaction of the hydroxylation of the proline and lysine. The lack of vitamin C is also caused in the synthesis of glycosaminoglycans: the content of hyaluronic acid in bone tissue increases several times, while chondroitin sulfate biosynthesis slows down.
Control questions
1. Describe calcium and phosphorus metabolism in the body.
2. What hormones are involved in the regulation of phosphorous calcium exchange?
3. What type of reception prevails in hormones regulating phosphorous calcium exchange?
4. How is the transformation of vitamin D in calcitriol?
5. List the symptoms observed in hypo- and hypercalcemia.
6. Name the main organic components of bone tissue.
7. What inorganic compounds are included in bone tissue?
8. Describe the bone formation process.
9. What factors affect the formation of bone tissue and its metabolism?
IN compact Bones: 20% - organic matrix, 70% - inorganic substances, 10% - water. IN spongy Bones: more than 50% - organic components, 33 - 40% - inorganic compounds, 10% - water.
Inorganic bone fabric composition . In the human body ~ 1 kg of calcium, 99% is in the bones and teeth. Most of the CA in the bones is constantly updated: per night the bones of the skeleton are losing and again get ~ 700 - 800 mg of sa. The inorganic components of bone tissue are presented:
crystals of hydroxyapatite Ca 10 (PO 4) 6 (OH) 2, which have the form of plates or chopsticks;
amorphous phosphate Ca 3 (PO 4) 2, which is considered a labile reserve of ions Ca and R.
At an early age, sa 3 (PO 4) 2 prevails, and in mature bones - hydroxyapatite.
Na +, Mg 2+, K +, Cl -, etc.
Organic bone fabric matrix: ~ 95% - Type I collagen. There are many free ε-NH 2 groups of Liz and oxilyzine, as well as related to the residuals of gray phosphates. The number of proteoglycans in mature dense bone is small. Among glycosaminglikans, chondroitine-4 sulfate prevails and less contains chondroitin-6-sulfate, keratan sulfate and hyaluronic acid; They are involved in ossification. Many citrate (up to90% of the total in the body): The citrate may form complex compounds with SA and P salts and thereby increases them in tissue to such a level in which crystallization and mineralization begins.
Throughout the body of the body, constant restructuring of bone tissue continues. It is believed that the bone cloth of a skeleton of a person is almost completely rebuilt every 10 years. The metabolism of bone tissue, admission, deposition and excretion of Ca and P are regulated by paratyrin, calcitonin, calcitrigol (1.25 (OH) 2-d 3) (repeat!). Parathirinactivates osteoclasts, mineral (in the 1st stage Ca) and organic components enter blood. Calcithonin suppresses the activity of these cells, and the rate of bone formation is growing. With lack vitamin D.participating in SA-SAT synthesis slows down the formation of new bones and remodeling (updating) of bone tissue. Chronic excess WIT.D leads to bone demineralization. VIT.A.: With the lack of incidence of bones, due to, probably, disorders of chondroitin sulfate synthesis; With hypervitaminosis - resorption of bones and fractures. Vit.s need for hydroxylation of pro and Liz; With a lack of: 1), abnormal collagen is formed, the mineralization processes are violated; 2) The synthesis of glycosaminglikanov is disturbed: the content of hyaluronic acid in bone tissue increases several times, and the synthesis of chondroitinatin sulfate slows down.
Chemical composition of the tooth.
The solid part of the tooth is represented by enamel, dentin and cement. The cavity of the tooth is made with a loose connective tissue - pulp.
Enamel –
the hardest fabric in the human body, due to the high content in it not organic substances (up to 97%). Healthy enamel contains 1.2% organic substances and up to 3.8% water, which can be free and related (in the form of a hydrated shell of apatite crystals).
Mineral base Code of Apatity crystals:
hydroxyapatite - 75%,
carbompatitis - 19%,
slopatitis - 4.4%,
fluoropatite - 0.66%,
neaspatite forms are less than 2%.
General formula for apatitis: a 10 (in 4) x 2, where
A - CA, CR, BA, CD, MG;
B - P, AS, SI;
X - F, OH, Cl, CO 3 2-.
Crystals of different teeth of unequal; Enamel crystals ~ 10 times more dentin crystals and bones. The composition of apatites may change. "Ideal" apatite - sa 10 (PO 4) 6 (OH) 2, i.e. Tental fatal, where the ratio of sa / p \u003d 1.67. This relationship may vary from 1.33 to 2.0, because Perhaps the flow of reactions of substitution:
Ca 10 (PO 4) 6 (O) 2 + Mg 2+ → Ca 9 Mg (PO 4) 6 (OH) 2 + CA 2+
Such substitution is unfavorable, because Reduces enamel resistance. Other replacement, on the contrary, to the formation of a substance with a greater dissolution resistance:
CA 10 (PO 4) 6 (O) 2 + F - → CA 10 (PO 4) 6 F (it) + it -
hydroxyifluorpaatite
However, when exposed to high concentrations F per hydroxyapatite, the reaction is different:
CA 10 (PO 4) 6 (O) 2 + 20 F - → 10 SF 2 + 6 PO 4 3- + 2
The resulting fluoride sa quickly disappears from the surface of the teeth.
In the crystal lattice of hydroxyapatite, vacant places can be, which increases the ability of crystals to surface reactions. Nr, if the tental hydroxyapatite has a common neutral charge, then the eight Calcium hydroxyapatite is negatively charged: (Ca 8 (PO 4) 6 (O) 2) 4- and is able to bind the counterions.
Each hydroxyapatitis crystal is coated with hydrate sheath (~ 1 nm). Penetration of various substances in the crystal of hydroxyapatitis goes in 3 stages:
1 Stage - ion exchange between a crystal solution, and a hydrate shell, in which phosphate, carbonate, citrate, Ca, Sr can accumulate as a result. Some ions (K +, Cl -) can easily enter the hydratt layer and leave it, other ions (Na +, F -), on the contrary, pass into the hydroxyapatite crystal. The 1st stage is a very fast process, lasts a few minutes, based on the diffusion process;
2 Stage - exchange of ions between the hydrate shell and the surface of the hydroxyapatitis crystal. Proceeds slower (several hours). The surfaces of the crystal ions are overclocked, go into the hydrated shell, others come up with their place, from the hydrate layer. In the surface of the hydroxyapatite crystal, phosphate, sa, f, carbonate, SR, Na penetrate;
3 Stage - the introduction of ions from the surface of the globe crystal, i.e. Intracrycrystalline exchange. Inside the crystal can penetrate CA, SR, phosphate, F. Long flows, days - months.
Thus, the crystals of hydroxyapatite are unstable, their composition and properties are changed depending on the solution that is washing the crystal. It is used in practical dentistry.
Most of the hydroxyapatitis crystals in the enamel in a certain way oriented and streamlined in the form of more complex formations - enamel prisms, each of which consists of thousands of thousands of millions of crystals. Enamel prism are collected in bundles.
Organic substancesenamels are represented by proteins, peptides, free amino acids (Gly, shaft, pro, ORD), fats, citrate, carbohydrates (galactose, glucose, mannose, glucuronic acid, fucose, xylose).
Enamel proteins are divided into 3 groups:
I - water-soluble proteins; Molecular weight - 20000, are not brought with mineral substances;
II - Calcium-binding protein (SA-Sat): Molecular weight 20000; 1 mol of Sat-Sat can bind 8 to 10 ions of SA and form an insoluble complex with Ca 2+ in a neutral medium by type of di- and tetramers weighing 40-80 thousand. Phospholipids are involved in the formation of Sat-Sat Agregates. In an acidic medium, the complex breaks down;
III - proteins that are not soluble in EDTA and HCl (even in 1N R-RE). Insoluble enamels in the amino acid composition are similar to collagen, but not identical to him: in the protein enamel less than in collagen, about and globes, there are almost no ODA, but many carbohydrate-related carbohydrates.
The role of protein: 1) surrounding apatite, protein prevents acid contact with them or softens its influence, i.e. delay the demineralization of this layer;
2) are a matrix for mineralization and remineralization (in the mechanism of biological integrity).
Offered functional and molecular model of the structure of enamel, in accordance with which the SA-SAT molecules, interconnected by calcium bridges, form a three-dimensional mesh; Ca at the same time can be free or included in the structure of hydroxyapatitis. This mesh is attached to the cable (frame, mild enamel skeleton), which is formed by insoluble protein. Functional groups of Cas Cons capable of tie, and this is phosphate in composition or phosphoserine or phospholipids associated with protein; Son-group depth, ASP, aminoocitrate, serve as centers (points) of nucleation during crystallization. Thus, proteins provide orientation during crystallization, strict orderliness, uniformity and sequence of enamel formation. The degree of mineralization depends on the savory, blood supply, the degree of Ca 2+ and phosphate, from the pH of the medium, etc.
Dentine
makes the main mass of the tooth. (The crown of the tooth is covered with enamel, root - cement). Composition: up to 72% - inorganic substances (mainly phosphate, carbonate, calcium fluoride), ~ 28% - organic substances (collagen) and water. Dentin is built from the main substance and the tubes passing in it, in which the processes of odontoblasts and the end nerve fiberspenetrating the pulp. The main substance contains collagen fibers assembled in bundles and a bonding substance in which there is a large amount of mineral salts. The dentin formation process occurs during the entire period of the functioning of the tooth in the presence of a viable pulp. Dentin, formed after the teething of the tooth, is called secondary. It is characterized by a smaller degree of mineralization and a large content of collagen fibrils. According to the dentine tubes, the dentine fluid can circulate and nutrients flow. The intercaltsene substance is represented by hydroxyapatite crystals, has high density and hardness. In the cytoplasm of odontoblasts a lot of fibrils, there are free ribosomes, lipid granules.
Each human bone is a complex organ: it occupies a certain position in the body, has its own form and structure, performs the function characteristic of it. All types of tissues take part in the formation of the bone, but bone tissue prevails.
General characteristic of human bones
The cartilage covers only the articular surfaces of the bone, outside the bone is covered with an assault, inside the bone marrow is located. The bone contains fatty tissue, blood and lymphatic vessels, nerves.
Bone It has high mechanical qualities, its strength can be compared with metal strength. The chemical composition of human living bone contains: 50% water, 12.5% \u200b\u200bof protein organic substances (Ossein), 21.8% of inorganic substances (mainly calcium phosphate) and 15.7% fat.
Bone types in shape Divide on:
- Tubular (long - shoulder, femoral, etc.; Short - phalanges of fingers);
- flat (frontal, dark, blade, etc.);
- sponge (ribs, vertebrae);
- mixed (wedge-shaped, cheekbone, lower jaw).
The structure of the bones of man
The main structure of the unit of bone tissue is osteon which is visible in a microscope at a small increase. Each osteon includes from 5 to 20 concentricly located bone plates. They resemble cylinders inserted into each other. Each plate consists of an intercellular substance and cells (osteoblasts, osteocytes, osteoclasts). In the center of Osteon there is a channel - Osteon channel; It takes vessels. Between the adjacent osteon are located insert bone plates.

Bone tissue form osteoblasts, Having mediating the intercellular substance and aligning it in it, they turn into osteocytes - the cells of the process-shaped form incapable of mitosis, with poorly pronounced organelles. Accordingly, the formed bone contains mainly osteocytes, and the osteoblasts are found only in the growth sites and regeneration of bone tissue.
The largest amount of osteoblasts is in an extra-thin, but dense coupling plate containing many blood vessels, nerve and lymphatic endings. The periosteum provides an increase in the bone in the thickness and nutrition of the bone.
Osteoclasts Contain a large number of lysosomes and are able to allocate enzymes than can be explained by the dissolution of the bone substance. These cells take part in the destruction of the bone. In pathological conditions in bone tissue, the number of them sharply increases.
Osteoclasts are in the process of the development of the bone: in the process of building the final shape of the bone, they destroy the occasional cartilage and even a newly formed bone, "correcting" its primary shape.
Bone structure: compact and spongy substance
On the pickle, the bone polls distinguish between its two structures - compact substance (bone plates are located tight and ordered), located superficially, and spongy substance (Bone elements are loose), lying inside the bone.

Such a bone structure fully corresponds to the basic principle of construction mechanics - with the smallest material of the material and high ease to ensure the maximum strength of the structure. This is confirmed by the fact that the location of tubular systems and basic bone beams corresponds to the direction of compression, stretching and twisting.
The structure of the bones is a dynamic reactive system, changing throughout the human life. It is known that people engaged in severe physical labor, the compact layer of the bone reaches relatively large development. Depending on the change in the load on separate parts of the body, the location of bone beams and the bone structure as a whole may vary.
Connection of human bones
All bone connections can be divided into two groups:
- Continuous connections, earlier development in philogenesis, fixed or low-modular function;
- interrupted compounds, later in development and more movable functions.
Between these forms there is a transitional - from continuous to a discontinuous or vice versa - polishetska.

The continuous bonding compound is carried out by means of connective tissue, cartilage and bone tissue (bone of the actual skull). The interrupted bonding compound, or the joint, is the younger formation of bone compounds. All joints have a common structure of the structure, which includes the articular cavity, the articular bag and the articular surfaces.
Articular cavity It is imposed conditionally, since it is normal between the articular bag and the articular ends of the bones of the void, but is liquid.
Mustic bag It covers the articular surfaces of the bones, forming a hermetic capsule. The articular bag consists of two layers, the outer layer of which goes into the periosteum. The inner layer allocates a liquid to the joint cavity, which plays the role of lubrication, providing free sliding of the articular surfaces.
Types of joints
The articular surfaces of the articular bones are covered with articular cartilage. Smooth surface The articular cartilage contributes to the movement in the joints. The articular surfaces in form and the magnitude are very diverse, they are customary to compare with geometric figures. From here I. the name of the joints in form: Character (shoulder), ellipsed (ray-cranky), cylindrical (ray-elbieva), etc.
Since the movements of the articious links are performed around one, two or many axes, the joints are also accepted by the number of axes of rotation. on multi-axis (spherical), biaxial (ellipsed, saddular) and uniaxial (cylindrical, block).
Depending on the the number of articious bones The joints are divided into simple, in which two bones are connected, and complex, in which more than two bones are combined.