The highest temperature in the fire. Blowtorch Flame Temperature Performance
Flame is an incandescent gaseous medium, consisting largely of partially ionized particles, in which chemical interaction and physicochemical transformations of fuel particles, oxidizer, impurity particles occur, accompanied by "glow" and heat release.
Sometimes in the scientific literature, a flame is referred to as a "cold / low-temperature plasma", since in fact it is a gas consisting of thermally ionized particles with a small charge (usually no more than +/- 2-3), while a "true" or high-temperature, plasma is a state of matter in which the nuclei of atoms and their electron shells exist separately.
The gaseous environment of the flame contains charged particles (ions, radicals), which determines the presence of electrical conductivity of the flame and its interaction with electromagnetic fields. On this principle, devices are built that are capable of muffling a flame, tearing it away from combustible materials or changing its shape with the help of electromagnetic radiation.
Flame color
Candle flame
The usual flame that we observe when burning a candle, the flame of a lighter or a match, is a stream of incandescent gases stretched vertically due to the force of Archimedes (hot gases tend to rise upward). First, the candle wick heats up and the paraffin wax begins to evaporate. Zone 1, the lowest, is characterized by a slight blue glow - there is a lot of fuel and little oxygen. Therefore, incomplete combustion of the fuel occurs with the formation of CO, which, oxidizing at the very edge of the flame cone, gives it Blue colour... More oxygen penetrates into zone 2 due to diffusion, further fuel oxidation occurs there, the temperature is higher than in zone 1, but it is still insufficient for complete fuel combustion. Zone 1 and Zone 2 contain unburned fuel droplets and coal particles. Due to strong heating, they glow. The evaporated fuel and its combustion products - carbon dioxide and water - hardly glow. In zone 3, the oxygen concentration is even higher. Afterburning of unburned fuel particles that glowed in zone 2 occurs there, so this zone almost does not glow, although there is heat.
Classification
Flames are classified by:
- aggregate state of combustible substances: flame of gaseous, liquid, solid and aerodispersed reagents;
- radiation: luminous, colored, colorless;
- the state of the environment fuel - oxidizer: diffusion, premixed media;
- the nature of the movement of the reaction medium: laminar, turbulent, pulsating;
- temperature: cold, low temperature, high temperature;
- propagation speeds: slow, fast;
- height: short, long;
- visual perception: smoky, transparent, colored.
In a laminar diffusion flame, 3 zones (shells) can be distinguished. Inside the flame cone there are: a dark zone (300-350 ° C), where combustion does not occur due to a lack of an oxidizing agent; luminous zone, where thermal decomposition of fuel and its partial combustion occurs (500-800 ° C); barely luminous area, which is characterized by the final combustion of decomposition products and max. temperature (900-1500 ° C). The flame temperature depends on the nature of the combustible substance and the intensity of the oxidant supply.
Flame propagation over a premixed medium (unperturbed) occurs from each point of the flame front along the normal to the flame surface. The value of such an NSRP is the main characteristic of a combustible medium. It represents the lowest possible flame speed. The NSRP values differ for various combustible mixtures - from 0.03 to 15 m / s.
The propagation of a flame through actually existing gas-air mixtures is always complicated by external disturbing influences caused by gravity, convective flows, friction, etc. Therefore real speeds flame spreads are always different from normal. Depending on the nature of combustion, the flame propagation speeds have the following ranges of values: during deflagration combustion - up to 100 m / s; with explosive combustion - from 300 to 1000 m / s; with detonation combustion - over 1000 m / s.
Oxidizing flame
It is located in the upper, hottest part of the flame, where combustible substances are almost completely converted into combustion products. In this area of the flame, there is an excess of oxygen and a lack of fuel, therefore, substances placed in this area are intensively oxidized.
Restoring flame
This is the portion of the flame closest to or just below the center of the flame. In this area of the flame there is a lot of fuel and little oxygen for combustion, therefore, if a substance containing oxygen is introduced into this part of the flame, then oxygen is taken away from the substance.
This can be illustrated by the example of the reduction reaction of barium sulfate BaSO 4. Using a platinum loop, BaSO 4 is taken and heated in the reducing part of the flame alcohol burner... In this case, barium sulfate is reduced and barium sulfide BaS is formed. Therefore, the flame is called regenerative and rocks, including in field conditions using a soldering tube.
Flame in zero gravity
Under conditions where the acceleration of gravity is compensated by centrifugal force, for example, when flying in the earth's orbit, the combustion of matter looks somewhat different. Since the acceleration of gravity is compensated for, there is practically no Archimedes force. Thus, under zero gravity conditions, combustion of substances occurs at the very surface of the substance (the flame does not stretch), and combustion is more complete. Combustion products are gradually and evenly distributed in the environment. This is very dangerous for ventilation systems. Powders are also a serious danger, therefore, in space, powdered materials are not used anywhere, except for special experiments with powders.
In a stream of air, the flame is drawn out and takes on its usual form. Due to the gas pressure under zero gravity, the flame of gas burners also does not outwardly differ from combustion under terrestrial conditions.
Candles create a celebration. They give light, warmth and comfort. However, for curious people, the candle flame has always been an object of study. What's going on in the flames? Why is it not uniform in color? What's the temperature inside? If you answer the questions briefly, for reference only, then about paraffin candle the following is known:
There are three main zones in the flame. The first zone is almost colorless, with a blue tint, the closest to the wick. This is the paraffin evaporation zone. Since oxygen does not penetrate here, gases do not burn here. The temperature is the lowest - about 600 ° С. In the second, brightest zone, combustion occurs. The temperature reaches 800-1000 ° C. The orange and red glow is caused by incandescent carbon particles. The third, outer zone is the hottest. Complete combustion of carbon takes place here and the temperature reaches 1400 ° C. Enough to get burned!
Interestingly, bundling the candles together can actually lower the flame temperature by about 200 ° C or 15%. This phenomenon can be explained by the presence of a large number of wicks inside the flame, which causes intensive evaporation of wax, which in turn displaces gases from the combustion zone, even before they have time to completely burn out. However, even such a drop in temperature cannot explain the fact that bundles of 33 candles, lit by the holy fire on Orthodox Easter, do not burn people. There can only be a psychological explanation, not a physical one.
Michael Faraday wrote that "The phenomena observed during the burning of a candle are such that there is not a single law of nature that would not be affected in one way or another." I would like to separately note his excellent research work, published in 1861 "The History of the Candle". It was published in Russian in the series “Kvant Library”, issue 2. The book is available on the Internet under the link History of the Candle. In English by reference M. Faraday, "The chemical history of a candle" Faraday was an amazing scientist. He studied physical phenomena selflessly, with love. He always found the easiest and affordable way presenting their results. Here are the lines from the introductory chapter of the book:
“Before I begin, let me warn you: despite the depth of our chosen subject and despite our honest intention to understand it seriously and at a truly scientific level, I want to emphasize that I am not going to address only trained scientists from among here present. I take the liberty of speaking to young people, and speaking as if I were a young man myself. This is what I have done before, and so, with your permission, I will continue to do so now. And although I realize with full responsibility that every word I utter is ultimately addressed to the whole world, such responsibility will not scare me away from speaking just as simply and easily with those whom I consider closest to me. "
Faraday's lectures were not dry and boring. They always contained poetry and the author's personal attitude to the subject. In the aforementioned scientific work on the candle, he writes:
“Compare the brilliance of gold and silver and even more brilliance precious stones- ruby and diamond - but neither one nor the other can compare with the radiance and beauty of a flame. And really, what kind of diamond can shine like a flame? Indeed, in the evening and at night, the diamond owes its sparkle to the very flame that illuminates it. The flame shines in the dark, and the brilliance contained in the diamond is nothing until the flame illuminates it, and then the diamond sparkles again. Only the candle shines by itself and for itself or for those who made it. "
Candle burning research continues at the present time. Despite the fact that it is very dangerous to experiment with fire on space stations, 80 candles were burned on the ISS Mir in 1996, and it turned out that a candle that completely burns out on Earth in 10 minutes can burn 45 minutes at the station. However, the flame was very weak and bluish, it could not even be filmed with a video camera, and in order to prove the existence of this flame, one had to introduce a piece of wax into it and film it melting. The combustion process in zero gravity can only be supported by molecular diffusion or artificial ventilation. Without ventilation, the heat radiation from the combustion center only cools it down and can eventually stop the process without leaving even smoke. Under normal conditions, the thermal radiation serves as a positive feedback that supports combustion. Therefore, to stop the fire in zero gravity, it is enough to turn off the ventilation and wait a little.
And in conclusion, we note that no matter how many new energy-saving light bulbs are invented in our time, the candle will remain the most beautiful, magical and attractive for people. Probably, natural combustion reflects all the same laws of harmony by which man was created and lives.
- Blowtorch fuels
- Flame temperature
The current generation of left-handed people rarely use a blowtorch, preferring an electric industrial hair dryer or a gas torch, which are much easier and safer to use. But even 40-50 years ago, a blowtorch was in almost every home workshop of a locksmith or car enthusiast, since it was the only tool capable of heating various materials to the desired temperature.

The blowtorch burns gasoline in the nozzle, giving out a fairly large jet of open flame.
But it is still not worth putting a blowtorch into junk in our age of scientific and technological progress. For example, it is almost impossible to ignite a gas burner in severe frost. With an industrial hair dryer, the situation is no better: it needs a constant source of electricity to work. And an old blowtorch does not care about all these difficulties.
The principle of combustion in a blowtorch
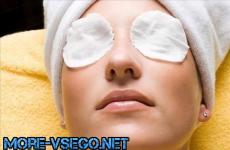
Blowtorch - heating device working on liquid fuel. Its peculiarity is that in the working tool, the burner, the vapors of the fuel filled into the lamp burn, and not the fuel itself. Entering the burner at a high speed, the jet of such vapors sucks in the air around the burner, thereby providing itself with a sufficient amount of oxygen.
Such self-sufficiency is very important, since for complete combustion of 1 kg of liquid hydrocarbon-based fuel, a certain amount of oxygen. In this case, complete combustion will be achieved, after which only carbon dioxide and water will remain from the fuel.
But if you just light a liquid fuel, such as gasoline, in open container, it will not completely burn out. This is indicated by the orange-red flame of similar burning centers, moreover, with a fair release of soot. But if air is artificially injected into such a combustion center, then the flame from orange-red will turn blue, practically without soot, and its temperature will increase significantly. Oxygen in the air will cause these changes.
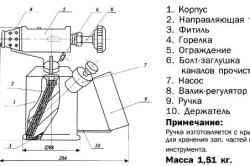
It is the principle of artificial enrichment of the flame with air, borrowed from gas lamps (so-called horns), that is the basis for the operation of a blowtorch. Moreover, such an air supply is regulated spontaneously: fuel vapors enter the burner, and the greater the intake, the more powerful the jet will be and, accordingly, the more air it will draw into itself.
Sometimes it happens that the jet draws in too much air, and the oxygen does not have time to completely burn out. In this case, the combustion temperature decreases markedly, since the excess air passing through the burner cools it. However, this only happens when using low-quality fuel. With normal filling of the burner with fuel vapors, it is impossible to draw in excess air into it for purely physical reasons.
Back to the table of contents
Blowtorch fuels
The versatility of a blowtorch is that it can work on almost any flammable liquid fuel: alcohol, kerosene, gasoline, diesel fuel, oil. But this does not mean at all that anything can be poured into each blowtorch.
Fuel must be of high quality. In addition, it must be borne in mind that an unsuitable type of fuel will very quickly clog the nozzle with its vapors. Today there are three types of blowtorches:
- kerosene;
- gasoline;
- alcohol.
The principle of the blowtorch remains in operation gas burner, therefore, some specialized sources also refer this device to blowtorches, highlighting it as a separate, fourth, type.
Refueling the lamp with another type of fuel that does not correspond to its design is strictly prohibited by the safety instructions. And this rule must be strictly observed. After all, kerosene poured into a gasoline "soldering iron" will make it a tool like a flamethrower. Getting into the burner, it will not have time to completely evaporate, therefore, not the vapors will burn, but the kerosene itself. Such a tool will not work normally.
It is even more dangerous to pour gasoline into a kerosene blowtorch. Gasoline evaporates much faster than kerosene, and its vapor pressure in the burner will be 6 times higher than the calculated one. When attempting to ignite, the vapors will explode, turning useful tool into a dangerous bomb. Therefore, if you use a kerosene blowtorch, you need to fill it only with pure kerosene, without any impurities, without using mixtures of kerosene with gasoline or other fuels.
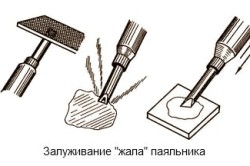
The situation is the same with a gasoline blowtorch. It should only be filled with clean gasoline. At the same time, the octane number of gasoline has practically no effect on the operation of the tool: neither on the speed of ignition, nor on the burning time, nor on the flame temperature. But when choosing a brand of gasoline, one should not forget that low-octane brands of various additives and impurities are much less, therefore, the nozzle will become much less dirty during operation.
Alcohol blowtorches have a small tank volume (only 200-300 ml), respectively, its combustion is very limited in time, so today craftsmen prefer to use gas burners instead.
Back to the table of contents
Flame temperature
There is a belief among a significant part of users that it is impossible to perform high-quality complex soldering with a blowtorch and that it is only suitable for heating frozen water pipes and sewer pipes, warming up the engine at low temperatures and for other similar work.
Such a reputation arose due to inattention to technical specifications tool or misunderstanding of the principle of work. The maximum temperature that a flame coming out of a blowtorch can give is 1100 ° C. Therefore, trying to melt iron, nickel or chromium with a blowtorch is hopeless. At the same time, its temperature is quite enough to melt lead, zinc, tin or aluminum.
In order not to waste time in vain, it is better to have metal melting data on hand:

A soldering iron is needed to perform minor repairs of electronic devices, but it is rarely used at home, so it is not cost-effective to buy it, you can use its substitutes.
- tin - 230 ° C;
- bismuth - 270 ° C;
- lead - 330 ° C;
- zinc - 420 ° C;
- magnesium - 650 ° C;
- aluminum - 660 ° C;
- bronze - 830 ° C;
- brass - 890 ° C;
- silver - 960 ° C;
- neodymium 1030 ° C;
- gold - 1060 ° C;
- copper - 1080 ° C;
- nickel - 1450 ° C:
- iron - 1540 ° C;
- titanium - 1660 ° C;
- platinum - 1770 ° C;
- chrome - 1900 ° C;
- molybdenum - 2620 ° C.
Based on these data, it is easy to determine which metal with the help of a blowtorch can be worked with without difficulty, with which it is possible, but with difficulty, and which work with which it is useless to undertake.
But it is also possible that even work with low-melting metals is unsuccessful. The reason for this is improper use of the lamp, or rather misunderstanding physical properties flame. After all, the flame coming out of the burner is not the same in its physical components.
Depending on the excess or lack of oxygen in it, it can have a different temperature.
Experts distinguish between three types of flame: reducing or normal; oxidative, formed with an excess of oxygen in the mixture and promoting the oxidation of metals, and carburizing, when there is an excess of fuel gases in the mixture. When processing the metal with the latter, its surface is saturated with carbon, which leads to a significant increase in its hardness, but at the same time to brittleness.
By itself, even the maximum flame temperature cannot serve as a guarantee of the quality of work performed with a blowtorch. When working with this tool, it is very essential have knowledge of the basics of soldering and especially work experience. If you do not possess either the first or the second, then it is better to seek help from a more experienced friend. Indeed, no matter how easy the lamp is in operation, due to the use of flammable explosive liquids in its operation, it has been and remains a source of increased danger.
Before tinning the soldering iron, you should find out what this procedure is and why it needs to be carried out. The bottom line is that as a result of rations, due to overheating, the tip of the soldering iron is oxidized and, accordingly, loses its ability to normally melt the solder.
In laboratory conditions, a colorless fire can be achieved, which can only be determined by the vibration of the air in the combustion area. Household fire is always "colored". The color of a fire is mainly determined by the temperature of the flame and what chemicals are burned in it. The high temperature of the flame allows the atoms to jump for some time to a higher energy state. When atoms return to their original state, they emit light at a specific wavelength. It corresponds to the structure of the electronic shells of this element.
Famous blue the light that can be seen when burning natural gas is due to carbon monoxide, which gives this hue. Carbon monoxide, a molecule composed of one oxygen and one carbon, is a byproduct of natural gas combustion.
Try sprinkling on the burner gas stove a little table salt - yellow tongues will appear in the flame. Such yellow-orange flame give sodium salts (and table salt, recall, this is sodium chloride). Wood is rich in such salts, so an ordinary forest fire or household matches burn with a yellow flame.
Copper imparts flames green shade. With a high copper content in the combustible substance, the flame has a bright green color, almost identical to white.
Green color and barium, molybdenum, phosphorus, antimony also give its shades to fire. IN blue colors the flame with selenium, and in blue-green- boron. Red flame will give lithium, strontium and calcium, violet - potassium, yellow-orange tint comes out when sodium is burned.
Flame temperature during combustion of some substances:
Did you know ...
Due to the property of atoms and molecules to emit light a certain color a method for determining the composition of substances was developed, which is called spectral analysis... Scientists study the spectrum that a substance emits, for example, when burning, compare it with the spectra of known elements, and thus determine its composition.
Fuel types. Combustion of fuel is one of the most common sources of energy used by humans.
There are several fuels by state of aggregation: solid fuel, liquid fuels and gaseous fuels. Accordingly, examples can be given: solid fuel is coke, coal, liquid fuel is oil and its products (kerosene, gasoline, oil, fuel oil, gaseous fuel are gases (methane, propane, butane, etc.)
An important parameter of each type of fuel is its calorific value, which, in many cases, determines the direction of fuel use.
Calorific value- this is the amount of heat that is released during the combustion of 1 kg (or 1 m 3) of fuel at a pressure of 101.325 kPa and 0 0 C, that is, under normal conditions. Expressed calorific value in units of kJ / kg (kilojoule per kg). Naturally, different types fuels with different calorific values:
Brown coal - 25550  Hard coal - 33920
Hard coal - 33920  Peat - 23900
Peat - 23900
- kerosene - 35000
- wood - 18850
- gasoline - 46000
- methane - 50,000
It can be seen that methane has the highest calorific value of the above-listed fuels.
In order to obtain the heat contained in the fuel, it must be heated to its flashpoint and, of course, with sufficient oxygen. In the process of a chemical reaction - combustion - is released a large number of warmth.
How the coal burns. Coal heats up, heats up under the influence of oxygen, thus forming carbon monoxide (IV), that is, CO 2 (or carbon dioxide). Then CO 2 in the upper layer of hot coals reacts with coal again, resulting in the formation of a new chemical compound - carbon monoxide (II) or CO - carbon monoxide... But this substance is very active, and as soon as a sufficient amount of oxygen appears in the air, the CO substance burns with a blue flame to form the same carbon dioxide.
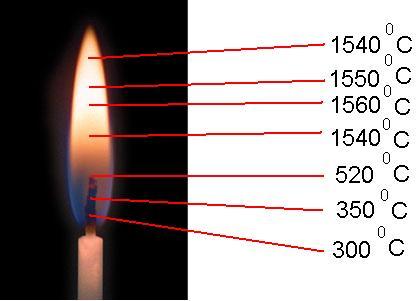
Probably ever asked ourselves the question, what is flame temperature?! Everyone knows that, for example, for some chemical reactions heating of the reagents is required. For such purposes, laboratories use a gas burner operating on natural gas having a wonderful calorific value... When burning fuel - gas, the chemical combustion energy is converted into thermal energy... For a gas burner, the flame can be depicted as follows:
The highest point of the flame is one of the hottest spots in the flame. The temperature at this point is about 1540 0 C - 1550 0 C
Slightly below (about 1/4 part) - in the middle of the flame - the hottest zone is 1560 0 C