Occupational safety in aviation. Elements of the theory of spontaneous combustion. Self-heating temperature. The difference between spontaneous combustion and spontaneous combustion and ignition - Fire extinguishing compounds
According to thermal theory, the self-ignition temperature is understood as the lowest temperature of a substance (material, mixture), at which a sharp increase in the rate of exothermic reactions occurs, ending in the occurrence of flaming combustion. In Fig. 2.3 such temperature is the temperature T s, corresponding to the point IN, in which the heat removal line q 2 touches the heat release line.
Temperature measurement T s in practice it is very difficult, which is due to the high rates of change in the temperature of the mixture during its self-heating. Therefore, the lowest temperature of the vessel wall or environment, at which, under given conditions, self-ignition of the substance occurs, i.e. T 0. This does not involve too much error.
Time from the moment the temperature in the flammable substance is established T 0 until the temperature is reached T s called induction period or auto-ignition delay time. The induction period for the same substance is not the same and strongly depends on the composition of the combustible mixture, temperature and pressure. The lower the heating temperature of a combustible substance during self-ignition, the longer the induction period. By
Therefore, the temperature of the environment or the walls of the vessel at which the induction period is longest is often taken as the auto-ignition temperature.
Below is shown the change in the induction period of methane-air mixtures depending on their composition and the temperature of the vessel:
|
Induction period, s |
|||
|
at 775 0 C…………………………………………… |
|||
|
at 825 0 C …………………………………………… |
|||
|
at 875 0 C…………………………………………… |
|||
When determining the auto-ignition temperature, it is impossible to measure the induction period; therefore, the induction period is taken to be the time from the moment the substance is heated until the flame appears. The induction period is of practical importance when a flammable substance is exposed to low-power ignition sources (sparks). When a spark hits a flammable mixture of vapors or gases with air, a certain volume of the mixture heats up and at the same time the spark cools. The ignition of the mixture in this case depends on the ratio of the induction period of the mixture and the cooling time of the spark. If the induction period is longer than the time it takes for the spark to cool to a temperature below the auto-ignition temperature, then the mixture does not ignite. If the induction period is less than the cooling time of the spark, the mixture ignites. Thus, a low-power spark can ignite a mixture with a short induction period and may not ignite a mixture with a long induction period.
Induction period solids differs from the period of induction of gas and dust mixtures. If the induction period for gas mixtures is tens and hundreds of seconds, then the induction period for solid flammable substances can be hours, days and months. At the temperature of self-ignition of a substance, combustion does not yet occur. It arises and develops at a combustion (flame) temperature significantly higher than the self-ignition temperature. For example, the self-ignition temperature of gasoline is 260 0 C, and the temperature of its flame is 1200 - 1300 0 C. The jump in temperature rise from 260 to 1200 0 C is the result of self-heating of the mixture of gasoline vapors and air.
The self-ignition temperature of a combustible substance is not a constant value. According to the thermal theory of self-ignition, this temperature depends on the rate of heat release and the rate of heat removal, which, in turn, depend on the volume of the combustible substance, its concentration, pressure and other factors.
In experiments to determine the self-ignition temperature, it was found that it changes not only with a change in the volume of the flammable substance, but also with the shape of the vessel (container) in which the substance is located. This is explained by the fact that with a change in the shape or size of the vessel, the specific surface area of the heat sink changes S/ V. In vessels of the same shape, the larger the volume of the vessel, the smaller it is. Consequently, as the volume of the vessel increases, the rate of heat removal decreases and, in accordance with this, the auto-ignition temperature should decrease. The self-ignition temperatures of liquid vapors in vessels of various volumes given below confirm this assumption:
|
Vessel volume, l……………….. |
||||||||
|
Self-ignition temperature |
||||||||
|
acetone……………………… |
||||||||
|
benzene……………………… |
||||||||
|
gasoline……………………… |
||||||||
|
diethyl ether………….. |
||||||||
|
kerosene……………………. |
||||||||
|
methyl alcohol………….. |
||||||||
|
carbon disulphide……………….. |
||||||||
|
toluene……………………… |
||||||||
the auto-ignition temperature decreases with increasing volume until the volume reaches a certain value (the shape of the vessel does not change); with a further increase in volume, the auto-ignition temperature remains constant.
Thus, the experiment shows that with a volume of more than 12 liters, the self-ignition temperature of the combustible mixture changes insignificantly. This is explained by the fact that in large volumes the combustible mixture does not spontaneously ignite in the entire volume at the same time, but in the part of it in which the most optimal conditions. Therefore, in a small volume of a combustible substance, a change in heat removal through the outer surfaces affects the change in the auto-ignition temperature, but in a large volume it does not.
The increase in the self-ignition temperature of a combustible substance with a decrease in volume is also not infinite. At a very small volume, the specific surface area of the heat sink becomes so large that the rate of heat release due to the oxidation of the combustible mixture, even at very high temperatures, cannot exceed the rate of heat removal, and self-ignition does not occur. Many devices designed to prevent the spread of combustion through gas mixtures (fire arresters) are designed and operate on this principle.
The simplest fire arrester is a protective mesh placed in a flammable gas mixture, which is broken into small volumes by the mesh. In this case, self-ignition cannot occur. The protective mesh is used in miners' lamps, as well as in small-diameter pipelines through which a mixture of air and petroleum product vapors is transported. The protective mesh cannot be used for mixtures of air with hydrogen, acetylene, carbon disulfide vapor, alcohols, ethers and other substances that have either a low auto-ignition temperature or a high heat of combustion. Under such conditions, the burning mixture, when passing through the burning mesh, does not cool below the auto-ignition temperature and continues to burn behind the mesh.
A larger specific surface area of the heat sink can be obtained not only by reducing the volume of the vessel, but also by giving it an appropriate shape. In Fig. 2.4 shows vessels different shapes, which contain equal amounts of the combustible mixture.
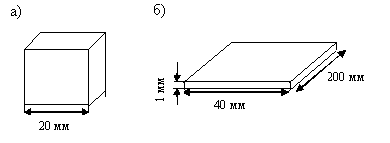
Rice. 2.4. Vessels of the same capacity with different heat removal rates
In the first vessel (cube) (Fig. 2.4, A) when heated, the mixture spontaneously ignites, in the second, which is a thin gap (Fig. 2.4, b), the mixture does not spontaneously ignite. This is explained by the fact that the second vessel has several times larger heat removal surface than the first.
> Elements of the theory of spontaneous combustion. Self-heating temperature. The difference between spontaneous combustion and spontaneous combustion and ignition
Proper organization fire prevention measures and extinguishing fires is impossible without understanding the essence of the chemical and physical processes that occur during combustion. Knowledge of these processes makes it possible to successfully fight fire.
Combustion is a chemical oxidation reaction accompanied by the release of a large amount of heat and usually glow. The oxidizing agent in the combustion process can be oxygen, as well as chlorine, bromine and other substances.
In most cases, during a fire, oxidation of combustible substances occurs with atmospheric oxygen. Combustion is possible in the presence of a substance capable of burning, oxygen (air) and an ignition source. In this case, it is necessary that the combustible substance and oxygen be in certain quantitative ratios, and the ignition source has the necessary reserve of thermal energy.
It is known that the air contains about 21% oxygen. The combustion of most substances becomes impossible when the oxygen content in the air drops to 14-18%, and only some flammable substances (hydrogen, ethylene, acetylene, etc.) can burn when the oxygen content in the air is 10% or less. With a further decrease in oxygen content, the combustion of most substances stops.
The combustible substance and oxygen are reacting substances and make up a combustible system, and the ignition source causes a combustion reaction in it. The source of ignition can be a burning or heated body, as well as an electric discharge with a reserve of energy sufficient to cause combustion, etc. spontaneous combustion ignition fire extinguishing
Combustible systems are divided into homogeneous and heterogeneous. Homogeneous systems are systems in which a flammable substance and air are uniformly mixed with each other (mixtures of flammable gases, vapors with air). The combustion of such systems is called kinetic combustion. Its speed is determined by the speed chemical reaction, significant at high temperature. Under certain conditions, such combustion may have the character of an explosion or detonation. Heterogeneous systems are systems in which the flammable substance and air are not mixed with each other and have interfaces (solid combustible materials and non-atomized liquids). During the combustion of inhomogeneous combustible systems, air oxygen penetrates (diffuses) through the combustion products to the combustible substance and reacts with it. This kind of combustion is called diffusion combustion, since its speed is determined mainly by the relatively slow process of diffusion.
For combustion to occur, the heat from the ignition source must be sufficient to convert combustible substances into vapors and gases and to heat them to the auto-ignition temperature. Based on the ratio of fuel and oxidizer, combustion processes of poor and rich combustible mixtures are distinguished. Lean mixtures contain an excess of oxidizing agent and lack a combustible component. Rich mixtures, on the contrary, have an excess of the combustible component and a deficiency of the oxidizing agent.
The occurrence of combustion is associated with the obligatory self-acceleration of the reaction in the system. The process of self-acceleration of the oxidation reaction with its transition to combustion is called self-ignition. Self-acceleration of a chemical reaction during combustion is divided into three main types: thermal, chain and combined - chain-thermal. According to thermal theory, the process of self-ignition is explained by the activation of the oxidation process with an increase in the rate of the chemical reaction. According to the chain theory, the process of self-ignition is explained by the branching of chemical reaction chains. In practice, combustion processes are carried out primarily by a combined chain-thermal mechanism.
Combustion is distinguished between complete and incomplete. With complete combustion, products are formed that are no longer capable of burning: carbon dioxide, sulfur dioxide, water vapor. Incomplete combustion occurs when air oxygen access to the combustion zone is difficult, resulting in the formation of incomplete combustion products: carbon monoxide, alcohols, aldehydes, etc.
Approximately the amount of air (m3) required for the combustion of 1 kg of substance (or 1 m3 of gas),
Heat of combustion of some substances: gasoline - 47,000 kJ/kg; air-dried wood -14,600 kJ/kg; acetylene - 54400 kJ/m3; methane - 39400 kJ/m3; carbon monoxide - 12600 kJ/m3.
By the heat of combustion of a flammable substance, you can determine how much heat is released during its combustion, combustion temperature, pressure during an explosion in a closed volume and other data.
The combustion temperature of a substance is determined both theoretical and actual. The theoretical combustion temperature is the temperature to which combustion products are heated, under the assumption that all the heat released during combustion is used to heat them.
The actual combustion temperature is 30-50% lower than the theoretical one, since a significant part of the heat released during combustion is dissipated into the environment.
High combustion temperature contributes to the spread of fire, with it a large number of heat is radiated into the environment, and intensive preparation of combustible substances for combustion takes place. Extinguishing a fire at high combustion temperatures is difficult.
When considering combustion processes, the following types should be distinguished: flash, combustion, ignition, spontaneous combustion, spontaneous combustion, explosion.
Flash- this is the rapid combustion of a combustible mixture, not accompanied by the formation of compressed gases.
Fire- the occurrence of combustion under the influence of an ignition source.
Ignition- fire accompanied by the appearance of a flame.
Flammability- the ability to ignite (ignite) under the influence of an ignition source.
Spontaneous combustion is a phenomenon of a sharp increase in the rate of exothermic reactions, leading to the combustion of substances (material, mixture) in the absence of an ignition source.
Self-ignition- This is spontaneous combustion accompanied by the appearance of a flame.
Explosion is an extremely rapid chemical (explosive) transformation of a substance, accompanied by the release of energy and the formation of compressed gases capable of performing mechanical work.
It is necessary to understand the difference between the processes of combustion (ignition) and spontaneous combustion (spontaneous combustion). In order for ignition to occur, it is necessary to introduce into the combustible system a thermal impulse having a temperature exceeding the self-ignition temperature of the substance. The occurrence of combustion at temperatures below the self-ignition temperature is referred to as the process of spontaneous combustion (self-ignition).
In this case, combustion occurs without introducing an ignition source - due to thermal or microbiological spontaneous combustion.
Thermal spontaneous combustion substances arise as a result of self-heating under the influence of a hidden or external heating source. Self-ignition is possible only if the amount of heat released during the auto-oxidation process exceeds the heat transfer to the environment.
Microbiological spontaneous combustion occurs as a result of self-heating under the influence of the vital activity of microorganisms in the mass of a substance (material, mixture). The auto-ignition temperature is an important characteristic of a flammable substance.
Auto-ignition temperature- this is the most low temperature a substance in which there is a sharp increase in the rate of exothermic reactions, ending in the occurrence of flaming combustion.
The auto-ignition temperatures of some liquids, gases and solids used in the engineering industry are given in Table. 1.
Table 1 Autoignition temperatures of some liquids
In addition to the auto-ignition temperature, flammable substances are characterized by an induction period or auto-ignition delay time. The induction period is the period of time during which self-heating occurs before ignition. The induction period for the same combustible substance is not the same and depends on the composition of the mixture, initial temperature and pressure.
The induction period is of practical importance when a flammable substance is exposed to low-power ignition sources (sparks). A spark entering a flammable mixture of vapors or gases with air heats a certain volume of the mixture, and at the same time the spark cools. Ignition of the mixture depends on the ratio of the induction period of the mixture and the cooling time of the spark. Moreover, if the induction period is longer than the spark cooling time, then the mixture will not ignite.
The induction period is adopted as the basis for classifying gas mixtures according to the degree of their ignition hazard. The induction period of dust mixtures depends on the size of dust particles, the amount of volatile substances, humidity and other factors.
Some substances can spontaneously ignite when at normal temperatures. These are mainly solid porous substances, mostly of organic origin (sawdust, peat, fossil coal, etc.). Oils distributed in a thin layer over a large surface are also prone to spontaneous combustion. This determines the possibility of spontaneous combustion of oily rags. The reason for spontaneous combustion of oiled fibrous materials is the distribution of fatty substances in a thin layer on their surface and the absorption of oxygen from the air. Oxidation of oil by atmospheric oxygen is accompanied by the release of heat. If the amount of heat generated exceeds heat loss to the environment, a fire may occur.
The fire hazard of substances prone to spontaneous combustion is very high, since they can ignite without any heat supply at an ambient temperature below the spontaneous ignition temperature of the substances, and the induction period of spontaneously combustible substances can be several hours, days and even months. The process of accelerating oxidation (heating the substance) that has begun can be stopped only if a dangerous increase in temperature is detected, which indicates great importance fire prevention measures.
Machine-building enterprises use many substances capable of spontaneous combustion. Iron sulfides, soot, aluminum and zinc powder, etc. can spontaneously ignite when interacting with air. They can spontaneously ignite when interacting with water alkali metals, metal carbides, etc. Calcium carbide (CaC2), reacting with water, forms acetylene (C2H2).
Combustion is an exothermic reaction that occurs under conditions of progressive self-acceleration. Combustion can occur both as a result of a chemical reaction of a compound and the decomposition of substances, not only when combined with atmospheric oxygen, but also with substances that contain it (for example, lime). The combustion of many substances can occur in an environment of chlorine, bromine vapor, and sulfur.
Combustion is divided into several types: outbreaks, ignition, inflammation, spontaneous combustion, spontaneous combustion.
Flash point is the lowest temperature of a concentrated substance at which, under special test conditions, vapor is formed above its surface, which is capable of flashing in the air from an ignition source; In this case, stable combustion does not occur.
Ignition is the rapid combustion of a flammable substance, which is not accompanied by the formation of compressed gas.
The liquid is not below the ignition temperature fire danger in case of short-term exposure to a flame, spark or hot body. If the liquid is heated to an ignition temperature or higher, then even a short-term exposure to a flame or spark on its vapor will inevitably cause it to ignite, and under certain conditions a fire may occur. Taking this into account, the flash point is taken as the basis for classifying liquids by degree fire safety. Liquids that can burn are divided into flammable (flammable) and combustible (GC) *.
The flammable substances most commonly found in aviation have the following flash points (in degrees Celsius):
* Flammable liquids include flammable liquids with a flash point not exceeding 61 ° C when determined in a closed crucible, or 66 ° C when determined in an open crucible. Liquids with a flash point higher than this are classified as flammable.
Depending on the flash point, you need to select safe methods transportation, storage and use of liquid for various purposes. At the flash point, stable combustion does not occur, but only the mixture of vapors and air formed above the liquid burns. If the temperature of the liquid is slightly higher than the flash point, the rate of evaporation will be open surface increases, and at the moment the mixture flares up, the liquid is able to continuously release steam in sufficient quantities for stable combustion. This temperature is called the ignition temperature. This is the lowest temperature of a substance at which, under special test conditions, the substance emits flammable vapor and gases at such a speed that when exposed to an energy source, they ignite.
Inflammation is a fire accompanied by the appearance of a flame.
In flammable liquids, the ignition temperature is 1-5 ° C higher than the flash point; moreover, the lower the flash point of the liquid, the smaller this difference. So, in benzene and acetone, which have a flash point below zero, this difference is 1 ° C; in flammable liquids, this difference reaches 30 ° C and above.
Spontaneous combustion- this is a sharp increase in the rate of exothermic volumetric reactions, which leads to a sharp increase in temperature and the occurrence of combustion of substances in the absence of an ignition source.
The thermal theory of self-ignition was first developed by S.N. Semenov and further developed in more detail by D. A. Frank-Kamenetsky and A. N. Todes.
The main provisions of this theory can be considered using the example of self-ignition of a mixture of flammable vapors or gases with air. At a low temperature T0 (for example, plus 20 ° C), the reaction between kerosene and air oxygen in the mixture practically does not occur, since there are no active oxygen molecules. In order for them to appear and the oxidation reaction to begin, the mixture must be heated to a higher temperature T1. To do this, place the container with the mixture in a medium that has a temperature of T1 (Figure 15.1a). After some time, the dishes and the mixture in it will heat up to temperature T1 and an oxidation process will occur in the mixture with the release of heat. The released heat q1 is transferred to the combustible mixture and is heated to temperature T1. However, as soon as the temperature of the mixture exceeds the temperature of the walls of the dish and the external environment, heat will begin to flow from the mixture to the walls of the dish and then to external environment. The amount of heat removed will be denoted by q2.

Rice. 15.1. A diagram explaining the process of spontaneous combustion of a combustible mixture:
a- heating the mixture due to the thermal energy supplied to it; b - thermal equilibrium; c - self-heating of the mixture and removal of heat from it into the environment
Further heating of the mixture will depend on the ratio of the rates of heat release and heat removal. If q1>q2 then the mixture, oxidizing, will heat up, and if q1 = q2, then the mixture will oxidize at any constant temperature, at which this relationship arose. Let us assume that the rate of heat release due to oxidation of the mixture exceeds the rate of heat removal. However, this is still not enough for the mixture to continue to heat up further, since with an increase in the temperature of the combustible mixture, the rate of heat release and heat removal does not increase equally. And if, with increasing temperature of the mixture, the rate of heat removal increases faster than heat release, then at a certain temperature of the mixture they will become equal (91 = 92) and subsequently heating will stop. This usually occurs at a low rate of oxidation of the combustible substance or at a large heat dissipation.
For example, steel shavings and sawdust also oxidize, so heat is released, but due to the low rate of oxidation, this does not always lead to combustion. In film oil paint An oxidation process occurs on the painted surface, but due to the very large surface of the increased heat dissipation, heating is not observed.
So, a mixture heated to temperature T2 due to reaction, oxidation, will gradually cool to temperature T1 (as soon as the concentration of reactants in the mixture begins to decrease). It follows from this that the process of oxidation of a combustible mixture heated to temperature T cannot go into combustion at a low reaction rate.
Let us increase the rate of oxidation of the mixture by heating it to temperature T3. The rate of heat removal will remain unchanged, since the surface of the vessel has not changed. This can lead to the fact that at a temperature of the combustible mixture Tu, the rate of heat release will constantly exceed the rate of heat removal and the mixture will be able to self-heat to a high temperature. When the temperature of the mixture reaches the combustion temperature, a flame will appear and combustion will occur. Consequently, a prerequisite for the spontaneous combustion process to occur is that the rate of heat release in the mixture exceeds the rate of heat removal.
So, thermal spontaneous combustion is the process of combustion that occurs as a result of self-heating of substances heated to a state during which the rate of heat release due to the oxidation reaction exceeds the rate of heat removal. Autoignition temperatures are usually taken as the temperature of the vessel walls at which spontaneous combustion occurs under given conditions.
The process of thermal self-ignition can be considered as a function of time (Fig. 15.2).
Let's place a flammable substance in air heated below the oxidation temperature. The temperature of the substance in this case will slowly increase (curve 1) and after some time will be equal to the air temperature T0. Because T0 is lower than the oxidation temperature, a flammable substance will behave like a non-flammable substance. If the air is heated to a temperature T1, which is higher than the oxidation temperature of this substance, the combustible substance will be heated (curve 2) to a temperature higher than T1, but then the temperature will begin to decrease.

Rice. 15.2. Graph of temperature changes of flammable substances when heated
Let's heat the air to temperature Ts> T1. Naturally, the rate of the oxidation reaction in this case will be much higher than in the previous one, and the temperature of the substance will rise above the air temperature Ts, reaching the value Tb, after which the temperature of the substance will rapidly increase to the combustion temperature. The process of spontaneous combustion is considered using the example of a gas mixture; it is typical not only for flammable vapors and gases. It applies to solids.
The auto-ignition temperature is not a constant value for the same flammable substance. It depends on the rates of heat release and heat removal, which in turn depend on the volume and shape of the combustible substance, its composition per unit volume, pressure and other factors. The limits for the auto-ignition temperature (in degrees Celsius) of some flammable and combustible substances are as follows:

In addition to thermal (with external heating), spontaneous combustion can also be microbiological and chemical.
Microbiological spontaneous combustion occurs due to self-heating in the mass of a substance under the influence of microorganisms. This phenomenon is observed during the storage of grain, hay, peat, coal, etc.
Chemical spontaneous combustion occurs as a result of the chemical interaction of substances when exposed to air and water (vegetable oil and animal fats, oils) in the presence of a large oxidation surface and low heat transfer to the environment.
The auto-ignition temperature in some flammable substances can exceed 500 ° C, and in others it can be below 16 ° C. All combustible substances with a self-ignition temperature can be conditionally divided into two groups: substances that have a self-ignition temperature higher than normal (16-25 ° C) and below. Substances of the first group are capable of spontaneous combustion only when they are heated to a particular temperature, substances of the second group are capable of spontaneous combustion without additional heating, since the environment has already heated them to the ignition temperature. Combustible substances pose an increased fire hazard because they may ignite under certain conditions. For example, aluminum in the form of powder as a result of oxidation is capable of self-heating and combustion. Self-heating can begin at normal ambient temperature or even lower, and end in combustion. If thin fabric, soaked in drying oil, form tightly, the heat that is generated during oxidation will not have time to dissipate in the air and will set the fabric on fire. However, if the same fabric is spread out rather than folded, then spontaneous combustion will not occur, since the heat that is released during the rapidly occurring oxidation process will be dissipated in the environment at a rate exceeding the rate of its formation.
Therefore, it is very important to know the substances that belong to the second group, since this allows us to determine the conditions for their storage and transportation special requirements which make it impossible for a fire to occur.
For example, as you know, there are mineral, vegetable and animal oils. Mineral oils oxidize in air only at high temperatures, and therefore do not spontaneously ignite. There are known cases of spontaneous combustion of a rag soaked in mineral aviation oils, which occurred as a result of impurities of vegetable oils entering it. Fats and oils containing organic compounds capable of spontaneous combustion.
The ability to spontaneously combust can be judged by the iodine number - the number of grams of iodine that combines with 100 g of oil. The more compounds there are in the oil, the more it adds iodine and, therefore, has a greater ability to spontaneously combust.
Drying oil with driers added to speed up drying, applied to fibrous materials, is capable of spontaneous combustion. Semi-natural and artificial drying oils are of little use or may spontaneously ignite. Oils, fats or drying oils that are in any closed container cannot ignite spontaneously, since the surface of their contact with air is very small. The ability of oils and fats to spontaneously combust increases significantly when the oxidation surface is significantly more surface heat transfer. Such conditions are created when oiled materials are arranged in piles, stacks, bags and adjacent to each other. The spontaneous combustion of oils and fats depends on the density of the packaging of the oiled material. Its ability to spontaneous combustion increases in the case of compaction up to a certain limit, after which it begins to decrease.
Ambient temperature also plays an important role in the process of self-ignition. The higher the air temperature, the smaller the volume of oiled material capable of spontaneous combustion, and less oil is needed. The probability of a fire in this case increases several times.
The lowest temperature at which spontaneous combustion of oils and fats is observed is 10-15 ° C. Cotton waste soaked vegetable oils(cloth wiping ends) may spontaneously ignite depending on environmental conditions. different terms(from several hours to several days).
The induction period (delay period for self-ignition) of substances that are capable of spontaneous combustion, in gases and liquids, oxidize in a gaseous environment, is very short. Almost the same period applies to solids that are in the state of aerosols.
Induction period for solids in the form of lumps different sizes may be long-lasting, since the surface was oxidized in this case. The rate of air diffusion onto the oxidation surface is also low.
Spontaneous combustion is called spontaneous combustion accompanied by the appearance of a flame. Combustion may be accompanied by fire and explosion.
According to flammability, substances and materials are divided into three groups: - fireproof are materials and structures that, under the influence of fire or high temperature, do not smolder or char (for example, oxidizers or substances that release flammable products when interacting with water, air oxygen or other with a friend);
Refractory materials and structures are those that, under the influence of fire or high temperature, become smoldering or charred and continue to burn and char in the presence of an ignition source, and after its removal these processes stop.
These include artificial materials, which, in addition to non-flammable minerals, have more than 80% by weight of organic fillers; structures made from fire-resistant materials, as well as from combustible materials protected from fire and high temperatures by non-combustible materials (wood coated with asbestos and roofing iron)
Combustible materials and structures are considered to be those that, under the influence of fire or high temperature, burn, smolder or char and burn after the source of ignition is removed. These include all organic materials that are not protected from fire or high temperatures.
Most flammable substances, regardless of their initial state of aggregation (solid, liquid, gaseous), when heated, transform into gaseous products and form flammable mixtures with air. Preparedness for fire is determined by the composition (concentration) of vapors, dust or gaseous products in them. There are minimum and maximum concentrations of flammable substances in the air, below and above which fire is impossible.
These concentrations are called lower and upper, respectively. concentration limits flammability.
Combustible gases and crushed solids (dust) can form flammable mixtures at any temperature. Solids as well as liquids form flammable mixtures only at certain temperatures. If the combustible mixture is prepared in accordance with the above conditions, then it can be considered that it is prepared for the fire that may occur if an ignition source appears.
The temperature limits of flame propagation (ignition) are those temperatures of a substance at which it saturated steam creates in an oxidizing environment concentrations equal to the lower (lower temperature limit) and upper (upper temperature limit) concentration limits of flame propagation, respectively.
The temperature limits of flame propagation are used: when developing measures to ensure fire and explosion safety of an object; for calculating fire and explosion hazards temperature conditions operation of technological equipment; estimates emergency situations associated with spills of flammable liquids; calculation of concentration limits of flame propagation. The value of temperature limits must be included in standards or in technical specifications for flammable substances. The possibility of combustion is mainly characterized by the flash, ignition, spontaneous ignition and spontaneous combustion temperatures.
Spontaneous combustion, the occurrence as a result of self-heating of flammable solid materials caused by self-acceleration of exothermic substances in them. reactions. Spontaneous combustion occurs due to the fact that the heat release during reactions is greater than the heat removal into the environment.
The onset of spontaneous combustion is characterized by the self-heating temperature ( Tсн), which is the minimum temperature under experimental conditions at which heat generation is detected.
When reaching during self-heating certain temperature, called the spontaneous combustion temperature ( T transport), combustion of the material occurs, manifested either by smoldering or flaming combustion. In the latter case T air intake is adequate to temperature ( T sv), which in firefighting is understood to occur when heated to a certain critical temperature. (see in firefighting) .
In principle, spontaneous combustion and self-ignition are similar in physical essence and differ only in the type of combustion; self-ignition occurs only in the form of flaming combustion.
In the case of self-heating (pre-explosive heating) develops within just a few degrees and is therefore not taken into account when assessing the fire and explosion hazard of liquids. During spontaneous combustion, the self-heating area can reach several hundred degrees (for example, for peat from 70 to 225 ° C). As a result, the phenomenon of self-heating is always taken into account when determining the tendency of solid substances to spontaneous combustion
Spontaneous combustion is studied by thermostatting the material under study at a given temperature and establishing the relationship between the temperature at which combustion occurs, the size of the sample and the time it is heated in the thermostat.
The processes occurring during spontaneous combustion of samples of combustible material are shown in the figure. At temperatures up to T sn (e.g. T 1) the material heats up without changes (no heat generation). Upon reaching T exothermic reactions occur in the material. The latter, depending on the conditions of heat accumulation (mass of the material, packing density of its atoms and molecules, duration of the process, etc.) can, after a period of slight self-heating upon the exhaustion of the material components capable of self-heating, end with cooling of the sample to the initial temperature of the thermostat (curve 1) or continue self-heat up to T import (curve 2). Area between T sn and T transport is potentially fire hazardous, below T sn -
safe.
Temperature change T in time t in thermostated samples of combustible material.
The possibility of spontaneous combustion of a material located in a potentially fire hazardous area is established using the equations:
Where T ambient - ambient temperature, °C; l- determining the size (usually thickness) of the material; t is the time during which spontaneous combustion can occur; A 1 , n 1 and A 2 ,n 2 - coefficient determined for each material according to experimental data (see table).
According to equation (1) for a given l find T environment in which spontaneous combustion may occur of this material, according to equation (2) - with a known T okr value t. At a temperature below the calculated T okr, or at t less than the time calculated by equation (2), spontaneous combustion will not occur.
Depending on the nature of the initial process that caused self-heating of the material, and the values T distinguish between chemical, microbiological and thermal spontaneous combustion

Chemical spontaneous combustion includes exothermic interaction of substances (for example, when concentrated HNO 3 gets on paper, sawdust and etc.). The most typical and widespread example of such a process is the spontaneous combustion of oily rags or other fibrous materials with a developed surface. Particularly dangerous are oils containing compounds with unsaturated chemical bonds and characterized by a high iodine number (cottonseed, sunflower, jute, etc.).
The phenomena of chemical spontaneous combustion also include the combustion of a number of substances (for example, finely crushed Al and Fe, hydrides of Si, B and some metals, organometallic compounds - organoaluminum, etc.) when they come into contact with air in the absence of heating. The ability of substances to spontaneously combust under such conditions is called pyrophoricity. The peculiarity of pyrophoric substances is that they T import (or T sv) below room temperature: - 200 ° C for SiH 4, - 80 ° C for A1 (C 2 H 5) 3. To prevent chemical spontaneous combustion, the procedure for joint storage of flammable substances and materials is strictly regulated.
Combustible materials are prone to microbiological spontaneous combustion, especially moistened ones, which serve as a breeding ground for microorganisms whose vital activity is associated with the release of heat (peat, sawdust, etc.). For this reason, a large number of fires and explosions occur when agricultural products (for example, silage, moistened hay) are stored in elevators. Microbiological and chemical spontaneous combustion is characterized by the fact that Tсн does not exceed normal values T okr and can be negative. Materials having T h above room temperature, capable of thermal spontaneous combustion
In general, many solid materials with a developed surface (for example, fibrous), as well as some liquid and melting substances containing unsaturated compounds deposited on a developed (including non-flammable) surface, are prone to all types of spontaneous combustion. The calculation of critical conditions for chemical, microbiological and thermal spontaneous combustion is carried out using equations (1) and (2). Methods of experimental determination T
The correct organization of fire prevention measures and fire extinguishing is impossible without understanding the essence of the chemical and physical processes that occur during combustion. Knowledge of these processes makes it possible to successfully fight fire.
Combustion is a chemical oxidation reaction accompanied by the release of large amounts of heat and usually a glow. The oxidizing agent in the combustion process can be oxygen, as well as chlorine, bromine and other substances.
In most cases, during a fire, oxidation of combustible substances occurs with atmospheric oxygen. This type of oxidizing agent is adopted in the following presentation. Combustion is possible in the presence of a substance capable of burning, oxygen (air) and an ignition source. In this case, it is necessary that the combustible substance and oxygen be in certain quantitative ratios, and the ignition source has the necessary reserve of thermal energy.
It is known that the air contains about 21% oxygen. The combustion of most substances becomes impossible when the oxygen content in the air drops to 14-18%, and only some flammable substances (hydrogen, ethylene, acetylene, etc.) can burn when the oxygen content in the air is 10% or less. With a further decrease in oxygen content, the combustion of most substances stops.
The combustible substance and oxygen are reacting substances and make up a combustible system, and the ignition source causes a combustion reaction in it. The source of ignition can be a burning or incandescent body, as well as an electrical discharge with a reserve of energy sufficient to cause combustion, etc.
Combustible systems are divided into homogeneous and heterogeneous. Homogeneous systems are systems in which a flammable substance and air are uniformly mixed with each other (mixtures of flammable gases, vapors with air). The combustion of such systems is called kinetic combustion. Its speed is determined by the speed of the chemical reaction, which is significant at high temperatures. Under certain conditions, such combustion may have the character of an explosion or detonation. Heterogeneous systems are systems in which the flammable substance and air are not mixed with each other and have interfaces (solid combustible materials and non-atomized liquids). During the combustion of inhomogeneous combustible systems, air oxygen penetrates (diffuses) through the combustion products to the combustible substance and reacts with it. Such combustion is called diffusion combustion, since its speed is determined mainly by the relatively slow process of diffusion.
For combustion to occur, the heat from the ignition source must be sufficient to convert combustible substances into vapors and gases and to heat them to the auto-ignition temperature. Based on the ratio of fuel and oxidizer, combustion processes of poor and rich combustible mixtures are distinguished. Lean mixtures contain an excess of oxidizing agent and lack a combustible component. Rich mixtures, on the contrary, have an excess of the combustible component and a deficiency of the oxidizing agent.
The occurrence of combustion is associated with the obligatory self-acceleration of the reaction in the system. The process of self-acceleration of the oxidation reaction with its transition to combustion is called self-ignition. Self-acceleration of a chemical reaction during combustion is divided into three main types: thermal, chain and combined - chain-thermal. According to thermal theory, the process of self-ignition is explained by the activation of the oxidation process with an increase in the rate of the chemical reaction. According to the chain theory, the process of self-ignition is explained by the branching of chemical reaction chains. In practice, combustion processes are carried out primarily by a combined chain-thermal mechanism.
Combustion is distinguished between complete and incomplete. With complete combustion, products are formed that are no longer capable of burning: carbon dioxide, sulfur dioxide, water vapor. Incomplete combustion occurs when air oxygen access to the combustion zone is difficult, resulting in the formation of incomplete combustion products: carbon monoxide, alcohols, aldehydes, etc.
Approximately the amount of air (m 3) required for the combustion of 1 kg of substance (or 1 m 3 of gas),
where Q is the heat of combustion, kJ/kg, or kJ/m 3.
Heat of combustion of some substances: gasoline - 47,000 kJ/kg; air-dried wood -14,600 kJ/kg; acetylene - 54400 kJ/m 3; methane - 39400 kJ/m 3; carbon monoxide - 12600 kJ/m 3.
By the heat of combustion of a flammable substance, you can determine how much heat is released during its combustion, combustion temperature, pressure during an explosion in a closed volume and other data.
The combustion temperature of a substance is determined both theoretical and actual. Theoretical is the combustion temperature to which combustion products are heated, under the assumption that all the heat released during combustion is used to heat them.
Theoretical combustion temperature
where m is the amount of combustion products formed during the combustion of 1 kg of substance; с - heat capacity of combustion products, kJ/ (kg*K); θ - air temperature, K; Q - calorific value, kJ/kg.
The actual combustion temperature is 30-50% lower than the theoretical one, since a significant part of the heat released during combustion is dissipated into the environment.
A high combustion temperature contributes to the spread of fire, during which a large amount of heat is radiated into the environment, and intensive preparation of combustible substances for combustion occurs. Extinguishing a fire at high combustion temperatures is difficult.
When considering combustion processes, the following types should be distinguished: flash, combustion, ignition, spontaneous combustion, spontaneous combustion, explosion.
A flash is the rapid combustion of a flammable mixture, not accompanied by the formation of compressed gases.
Fire is the occurrence of combustion under the influence of an ignition source.
Ignition is a fire accompanied by the appearance of a flame.
Flammability - the ability to ignite (ignite) under the influence of an ignition source.
Spontaneous combustion is a phenomenon of a sharp increase in the rate of exothermic reactions, leading to the combustion of substances (material, mixture) in the absence of an ignition source.
Spontaneous combustion is spontaneous combustion accompanied by the appearance of a flame.
An explosion is an extremely rapid chemical (explosive) transformation of a substance, accompanied by the release of energy and the formation of compressed gases capable of producing mechanical work.
It is necessary to understand the difference between the processes of combustion (ignition) and spontaneous combustion (spontaneous combustion). In order for ignition to occur, it is necessary to introduce into the combustible system a thermal impulse having a temperature exceeding the self-ignition temperature of the substance. The occurrence of combustion at temperatures below the self-ignition temperature is referred to as the process of spontaneous combustion (self-ignition).
In this case, combustion occurs without introducing an ignition source - due to thermal or microbiological spontaneous combustion.
Thermal spontaneous combustion substances arise as a result of self-heating under the influence of a hidden or external heating source. Self-ignition is possible only if the amount of heat released during the auto-oxidation process exceeds the heat transfer to the environment.
Microbiological spontaneous combustion occurs as a result of self-heating under the influence of the vital activity of microorganisms in the mass of a substance (material, mixture). The auto-ignition temperature is an important characteristic of a flammable substance.
The autoignition temperature is the lowest temperature of a substance at which a sharp increase in the rate of exothermic reactions occurs, ending in the occurrence of flaming combustion.
The auto-ignition temperatures of some liquids, gases and solids used in the engineering industry are given in Table. 28.
Table 28 Autoignition temperatures of some liquids
| Substance | Self-ignition temperature, °C |
Phosphorus white |
20 |
Carbon disulfide |
112 |
Celluloid |
140-180 |
Hydrogen sulfide |
246 |
Petroleum oils |
250-400 |
| 250 | |
Gasoline A-76 |
255 |
| 380-420 | |
Coal |
400 |
Acetylene |
406 |
Ethanol |
421 |
Charcoal |
450 |
Nitrobenzene |
482 |
| 530 | |
| 612 | |
| 625 | |
Carbon monoxide |
644 |
| 700 |
In addition to the auto-ignition temperature, flammable substances are characterized by an induction period or auto-ignition delay time. The induction period is the period of time
during which self-heating occurs until ignition occurs. The induction period for the same combustible substance is not the same and depends on the composition of the mixture, initial temperature and pressure.
The induction period is of practical importance when a flammable substance is exposed to low-power ignition sources (sparks). A spark entering a flammable mixture of vapors or gases with air heats a certain volume of the mixture, and at the same time the spark cools. Ignition of the mixture depends on the ratio of the induction period of the mixture and the cooling time of the spark. Moreover, if the induction period is longer than the spark cooling time, then the mixture will not ignite.
The induction period is adopted as the basis for classifying gas mixtures according to the degree of their ignition hazard. The induction period of dust mixtures depends on the size of dust particles, the amount of volatile substances, humidity and other factors.
Some substances can spontaneously ignite when at normal temperatures. These are mainly solid porous substances, mostly of organic origin (sawdust, peat, fossil coal, etc.). Oils distributed in a thin layer over a large surface are also prone to spontaneous combustion. This determines the possibility of spontaneous combustion of oily rags. The reason for spontaneous combustion of oiled fibrous materials is the distribution of fatty substances in a thin layer on their surface and the absorption of oxygen from the air. Oxidation of oil by atmospheric oxygen is accompanied by the release of heat. If the amount of heat generated exceeds heat loss to the environment, a fire may occur.
The fire hazard of substances prone to spontaneous combustion is very high, since they can ignite without any heat supply at an ambient temperature below the spontaneous ignition temperature of the substances, and the induction period of spontaneously combustible substances can be several hours, days and even months. The process of accelerating oxidation (warming up the substance) that has begun can be stopped only when a dangerous increase in temperature is detected, which indicates the great importance of fire prevention measures.
Machine-building enterprises use many substances capable of spontaneous combustion. Iron sulfides, soot, aluminum and zinc powder, etc. can spontaneously ignite when interacting with air. Alkali metals, metal carbides, etc. can spontaneously ignite when interacting with water. Calcium carbide (CaC2), reacting with water, forms acetylene (C 2 H 2 ).






