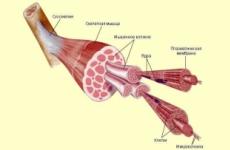
Fire hazard of solid combustible materials and dust
Classification of solid combustible materials (TGM)
In accordance with GOST 12.1.044-89, "fire hazardousness of substances and materials" are solid, the materials, the melting point or decomposition of which exceed 50 ° C, as well as substances that do not have melting points (wood, fabric, etc.).
TGM can be classified by several features:
- on chemical composition,
- by behavior when heated.
TO hydrocarbons Believe natural, artificial and synthetic polymer materialswhich includes carbon, hydrogen, nitrogen and oxygen. According to the structure, hydrocarbons are the materials of a homogeneous structure.
In a separate subgroup include natural organic substances, the basis of which is cellulose. These include polymeric materials of plant origin (wood, cotton, etc.), which, in contrast to artificial and synthetic polymers, are not homogeneous materials, but a mixture of natural polymers. Behavior in a fire conditions of all herbal materials seems to be, and for this reason they are united in one group - cellulose-containing materials.
Elementorganic connections - Organic substances that include elements such as sulfur, phosphorus, silicon, halides and metals. In a fire, elementorganic compounds form particular toxic substances and for this reason they are allocated to a special group.
Inorganic solid combustible substances - These are metals and non-metals. Almost all metals under normal conditions are oxidized in air. But only those that can ignite the air from an open source of ignition of medium power and sole after removing it are believed to be combustible. The most combustible includes alkaline and alkaline earth metals.
Nemetallam include phosphorus, arsenic, silicon, sulfur. The mechanism of their ignition is largely reminiscent of the peculiarities of the combustion of metals.
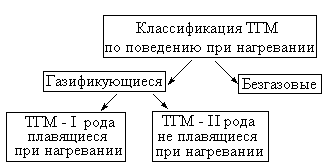
As can be seen from the scheme, all solids under behavioral behavior can be divided into two classes: gapeless and gasifant when heated.
The overwhelming majority of condensed substances belong to the second class. When heated, they are gasified, after which a homogeneous combustion of gasification products is carried out. In turn, the gasifying TGM are divided into two large groups by how they go to the vapor state. Solid combustible substances that pass into the gaseous state through the liquid phase (in conditions of high temperature melted), it is customary called TGM first kind.
The process of ignition of TGM 1st genus repeats the process of preparing and igniting combustible liquids. Their burning flows in homogeneous mode.
Solid combustible materials that pass into the steamed state bypassing the liquid phase due to sublimation or thermal destruction of molecules, it is customary called TGM Second Roda. With burning substances of this group, both homogeneous and heterogeneous combustion regime are possible.
General laws of ignition and burning TGM
The processes of the occurrence and development of combustion for solid combustible materials have a lot in common with the processes of combustion of gases and liquids studied by us. However, in addition, there are also a number of features due to aggregate state and differences in the structure.
Consider the mechanism of ignition of TGM. Upon contact with TGM with heated to high temperatures, heat exchange arises, while the following processes occur with the material:
- Heating the surface layer to the phase transition temperature (melting or thermal decomposition). If this is the material of plant origin, then moisture is beginning to evaporate from it.
- Further heating leads to the beginning of the phase transition. If it is the TGM of the 1st genus, then melting and the transition of the material in the liquid phase, then the melt heating to the boiling point or decomposition. If this is the material of the 2nd kind - the process of sublimation or decomposition is immediately beginning with the separation of volatile products.
- The formation of a combustible steam mixture and its preheating.
- Self-ignition of the stead-air mixture followed by burning.
Thus, if the thermal stream coming to the surface when burning the liquid is spent only for heating and evaporation of the liquid phase, then for solidsIn addition, melting and decomposition costs are needed.
At each stage proceeds physico-chemical processesthat define the state of the system. These stages correspond to the following zones:
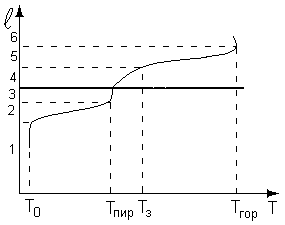
where T 0, T PIR, T s, T Mountains - the temperature of the initial, pyrolysis temperature, the ignition temperature, the combustion temperature, respectively.
- source zone;
- zone of preheating of the material to the temperature of physico-chemical transformations;
- it is a phase transition in which melting or decomposition of the material;
- zone of the formation of a combustible mixture and its heating to the ignition temperature;
- the flame front zone, where the main part of thermal energy is released and the maximum temperature is observed;
- the zone of combustion products, where the reaction products are mixed with cold air.
Thus, the process of burning most TGM begins with a homogeneous regime. The combustion is characterized by a high propagation rate, powerful convective streams and radiation.
The ignition time of TGM depends on the rate of formation above the surface of the material of the volatile components in a concentration exceeding the lower CRCR. The process of formation of volatile components comes with energy costs and for materials of different composition begins at different temperatures and proceeds with different intensity. The ability of the material to resist heating without changing the chemical structure is called thermal durability of material.
Spreading flame on TGM surface
After the ignition of TGM, the flame front is moving over the surface. The spread of burning flows through the transfer of heat from the combustion zone to another non-affordable sections of the material. Heat transmission is carried out due to radiation, convection and thermal conductivity. Depending on the conditions of combustion, the ratio of the amount of heat coming by these types of heat transfer may be different. Therefore, the rate of flame propagation over the TGM surface depends on the conditions of burning.
The most effect on the rate of flame spread over the TGM surface is the following factors:
- the nature of the material, its physico-chemical properties (the rate of formation of volatile products);
- moisture material;
- orientation of the sample in space;
- speed and direction of air flows;
- initial material temperature;
- geometric sample dimensions (thickness, dispersion).
The combustion of cellulose-containing materials
Cellulose - This is a high-molecular weight polysaccharide consisting of glucose molecules.
Consider behavior when heating wood as the most common combustible material.
The combustion of wood is significantly different from the combustion of liquids and gases, and can occur immediately in several modes - homogeneous and heterogeneous. Therefore, when combining wood, two phases can be distinguished: 1) homogeneous (i.e. fiery) combustion of gaseous decomposition products and 2) heterogeneous combustion of the resulting solid carbon residue.
The fiery burning stage takes a shorter period of time, but it allocates about 55-60% of all energy. The speed of heterogeneous combustion is determined by the speed of air intake to the surface.
Smoldering
Smoldering - Flare burning of fibrous and porous materials, which, when heated, form solid carbon residue. This is a special combustion regime, when combustible gases formed as a result of pyrolysis are not lit, and only heterogeneous burning of carbonaceous residue (surface oxidation) occurs. The calcination occurs due to oxygen contained in the pores of the material.
To materials that can target, a wide range of materials of plant origin (paper, cellulose tissues, sawdust), latex rubber, some types of plastics (polyurethane foam, foamoplanoplasts). Materials that can melt or decompose a little carbon residue are not capable of degeneration.
Dust burning
Dust - a colloidal system consisting of a solid dispersed phase and a gaseous dispersion medium, i.e. It is a solid, dispersed (thinly gridden) in a gaseous medium.
The dispersed phase can consist of particles of the same value ( monodisperse system) or particles of different quantities ( polydisperse system). All industrial dust polydisperse.
Depending on the average particle size, dust can be long in suspension or immediately settle after a short-term transition to a weighted state.
The dispersion system representing dust suspended in the air is called aerosol. The axial dust is called aergel.
Even in the inspected state, each separate particle of fragmented substance from all sides is surrounded by a gas (air) shell.
Aerosols in their properties occupy an intermediate position between the aerogel and a homogeneous gas-air mixture. As well as aerogels are heterogeneous dispersed systems with the same solid phase, and their behavior is determined. physico-chemical properties This solid phase. With gas-air mixtures of aerosols, the fact that the burning of most of them proceeds with an explosion, and they are characterized by many parameters typical of gas mixtures.
From the properties of dust, which determine their fire danger, are the most important: dispersion, chemical activity, adsorption capacity, leaning to electrification.
Features of the combustion of Aergel
The main parameters characterizing the fire danger of Aergel are the ignition and self-ignition temperature.
In general, the combustion of dust in the settling state is largely reminiscent of the combustion of solid fuel, from which this dust is obtained. A distinctive feature Aergel is HIS ability to move to a weighted state. When heated, everything proceeds preparatory processescharacteristic of solid combustible materials, but the rate of their flow is higher, which is explained by the developed surface, increased chemical activity, reduced thermal conductivity of the material as a result of grinding, increased dust adsorption capacity. This causes a smaller period of inflammation induction, a greater rate of combustion spread, as well as an increased tendency to self-burning compared to the initial material from which the dust is obtained.
Oxidative processes proceed simultaneously both on the surface of the dust layer and in its depth. In this case, oxygen adsorbed on the surface of the material takes part in the reaction. The rate of oxidation processes under the layer gorry dust an order of magnitude lower than on the surface as a result the burning in the thicker of dust sediments can go to the radiation mode. Glowing dust is a greater danger, since 1) the distinguished combustible decomposition products can accumulate in closed volumes, and the combustion of diffusion can go to kinetic; 2) Even with a weak shaking (turbulence), the smoldering mass can be self-splash due to a sharp fifth of oxygen and cause an explosion of zealous dust ..
Features of the burning of aerosol
Aerosols are ignited and lit like gas-air mixtures. Therefore, their fire hazard is characterized by the same parameters as gas-air mixtures: the CRCR, the minimum ignition energy, the maximum pressure of the explosion.
The tendency of aerosols to coagulation (sticking) and precipitation significantly distinguishes them from gas-air mixtures. This property causes high Ignition Energy (two orders of magnitude higher) than for gas mixtures.
If the spread of the flame in gas mixes due to the heating of the cold mixture due to thermal conductivity, the spread of the flame in dusty mixtures occurs due to women of a cold mixture by radiationemitted by the front of the flame.
The flame ignition and spread in the aerosol occurs only if the concentration is in the range of concentration limits of ignition.
The smallest concentration of dust in the air at which the mixture is capable of ignited from the ignition source, followed by the spread of burning on the entire volume of the mixture, is called the lower concentration limit of flame spread.
The upper concentration limit of flame propagation for dust also exists, and can be defined in laboratory conditions, but in practice it is not used, this is due to the fact that the constant existence of aerosol concentrations is above the upper limit, when the ignition is excluded, it is impossible and there will always be such a point in time, When, as a result of precipitation, the concentration of dust will be in an explosive range.
In the state of the aerosol, dust can ignite and burn in kinetic mode, i.e. With a blast, therefore, the main parameter of the fire hazard is taken by the NKPR. In the midst of the state, dust can be self-propagated and self-turn, for this to evaluate fire hazard properties Aergels use self-ignition temperature T SV.
All combustible dust can be divided into two groups and four classes:
The first group is explosive dust. Dust capable of kinetic burning and having a lower concentration flame proliferation limit up to 65 grams per cubic meter inclusive.
Grade 1 - the most explosive dust with the NKPP 15 g / m and below;
2 class - explosive dust with the NKPR from 15 and to 65 g / m;
Second group - fire hazardous dust
Grade 3 - the most fire-hazardous dust with T with not higher than 250 ° C;
4th grade - fire hazard dust with T SV above 250 ° C.
The NKPRP dusty systems depends on a number of factors, the main of which are:
- power out;
- dust humidity;
- ash content of material;
- the content of volatile components;
- the content of non-combustible gases;
- dust dispersion.
Scale of the approximate determination of the strength of the wind
| Wind | Wind speed, m / s | The observed action of the wind |
| Calm | 0-0,5 | Smoke rises shepherd or almost stuck. Leaves are stationary |
| Quiet | 0,6-1,7 | Flugger movements are invisible |
| Easy | 1,8-3,3 | The blow felt face. Leaves rustle |
| Weak | 3,4-5,2 | Leaves and thin branches of trees all the time pegs. Light flags flush |
| Moderate | 5,3-7,4 | Dust rises. Thin branches of trees are moving |
| Fresh | 7,5-9,8 | Thin trunks of trees are swinging, waves with scallops appear on the water |
| Strong | 9,9-12,4 | Thread bunches of trees are swinging, Telephone Wires |
| Strong | 12,5-15,2 | Tree trunks are swinging, big branches bend |
| Very strong | 15,3-18,2 | Thin branches and dry bums of trees break |
| Storm | 18,3-21,5 | Small destruction. Waves on the sea are covered by foam |
| Heavy storm | 21,6-25,1 | Significant destruction. Trees break off roots |
| Cruel storm | 25,2-29 | Big destruction |
| Hurricane | Above 29. | Catastrophic destruction |
Heat transmission B. environment It is carried out simultaneously in three ways: due to thermal conductivity, convection and radiating.
Thermal radiation, especially with outdoor fires, creates difficulties for the subsidy of personnel to the borders of the combustion. When exposed to a thermal pulse of 0.25 cal / cm * with for 3 minutes, pain sensations appear on unprotected skin.
SPRAVOCHMK_SPAS_5.QXP 05.06.2006 14: 50- ^ Rada 11
Under the temperature of open outer fires, it is necessary to understand the temperature of the flame, and the internal - mid-payable temperature of the mixture of combustion products with air in the amount of burning room.
The absolute values \u200b\u200bof the temperature of the outer fires are higher than the internal. It depends on the size of the combustion zone, the characteristics of combustible substances, fuel loading, the specific heat of the fire, the volume-planning solutions of the object (buildings), the conditions of gas metabolism and other factors. Fire temperature when burning different materials shown in table. 2.4.
Table 2.4./2/
With simultaneous burning of heterogeneous substances and materials, the average temperature of the fire temperature is determined by the weight share of the loading of these materials. Indoors big height The rate of formation of the maximum temperature is much higher than in low rooms. Fires in cellars, vessel holds, cable tunnels, drying chambers and other
SPRAVOCHMK_SPAS_5.QXP 05.06.2006 14: 50- ^ Rada 12
weightly closed rooms are characterized by a higher fire temperature, since they are limited to the transmission of heat in them with convection flows and accumulates it.
The fire temperature is not the magnitude of constant. It changes in time and space. The change in the temperature of the fire in time and space is called the temperature regime of the fire.
With internal fires under the temperature regime, it is necessary to understand the change in the mid-paying temperature in time, with the external - in time and the space of the heat influence zone to the safe boundaries.
The temperature distribution on the fire in height and in terms of occurs unevenly. The maximum temperature is formed in the burning zone, and the minimum - as the heat exposure zone is removed from it (the boundary is located there, where the temperature of the combustion products does not exceed 50-60 ° C). As the temperature is removed from the burning zone, the temperature decreases at the expense of the heat exchange occurring in the environment.
The temperature of the fire can be determined by measurement using thermocouples, optical and radiation pyrometers, by calculating heat-containing flue gases, according to the characteristic external signs of heating of bodies, structures, materials (melting, color of closer, etc.). The temperature of the flame during burning and the melting point of some substances is given in Table. 2.5 and 2.6.
High temperature in burning areas and thermal exposure may cause deaths of people and animals, cause heating with flammable materials, their ignition, deformation and collapse building structures, to have a significant impact on the development and environment of the fire, create complex conditions For the implementation of fighting for fire extinguishing.
Man at a temperature of 80-100 ° C in dry air and 50-60 ° C in wet can be without funds special protection A couple of minutes. Higher temperature and long-term stay of people in the zone of harmful thermal exposure can lead to burns, thermal impacts, loss of consciousness and even a deadly outcome.
The scientific theory of burning was first developed by M.V. Lomonosov in 1756 Currently, the generally accepted theories of combustion are the peroxidation theory of oxidation of academician A.N. Bach, developed by him in 1897, and chain theory of academician N.N. Semenova, developed in 1927
According to the peroxidation theory of oxidation, as a result of the interaction of an oxidized substance with oxygen, peroxide is formed. The excited oxygen molecules, the energy of which is higher than the average energy of the substance molecules are entering the reaction. This energy
A.N. Bach called the activation energy. Under the action of this energy, the oxygen molecule is activated to the active state, which is considered as a break of one of two bonds in the oxygen molecule.
Molecules can be activated under the action of energy. different species. Thus, the activation of the chlorine molecule occurs under the action of light energy, and oxygen molecules - under the action of thermal energy. The group is, in which atoms are weaker than in a free molecule, connecting with an oxidized substance, forms peroxide - a strong oxidizer.
The chain theory of oxidation develops and complements the peroxidation and allows you to explain the kinetic side of the phenomenon and the reasons for the acceleration of the process, and the activation paths of the reacting substances.
It is known, for example, that a mixture of hydrogen and chlorine, cooked in the dark, explodes into the light. Primary chain reaction
it is the decay of chlorine molecules to atoms when absorbing a quantum of light. The chlorine atom reacts with a hydrogen molecule, forming a hydrogen atom and a HCl molecule. The hydrogen atom reacts with a chlorine molecule, regenerating the chlorine atom.
Consequently, the formation of a single chlorine atom causes a chain of reactions that stop when as a result of recombination or reaction with an admixture is eliminated by an active center - a hydrogen or chlorine atom.
The burning is a chemical oxidation reaction accompanied by excretion. large number Heat and usually with a glow.
Fire - uncontrolled burning, which is coming out of a special focus and causes material damage.
Usually, the burning takes place in the air, and oxygen appears as an oxidant. However, there are a number of substances that can burn, connecting with other oxidizing agents. For example, acetylene burns in chlorine, magnesium - in carbon dioxide, phosphorus flashes, entering into a reaction with chlorine and bromine, etc. Acetylene, nitrogen chloride and a number of other gases during compression can explode, resulting in a decomposition of a substance with the release of light and heat. Thus, the combustion process may occur not only when chemical reaction Compounds, but also with decomposition reaction.
Chemical combustion processes are usually accompanied by physical processes of combustible substance in a liquid and gaseous state. For example, wax, paraffin and some other substances under the influence of heat are first transformed into a liquid, and then into pairs, which burns flame outside the combustible substance. The flammable and combustible fluids themselves are not lit, and their pairs are burning on the surface under the influence of heat.
For combustion in the air of the combustible substance, the presence of oxygen is necessary (at least 14-15% to the air volume) or other oxidizing agent and temperature at which it can burn. The combustion can occur not only due to air oxygen, but also due to oxygen contained in other
substances and easily distinguished from them (peroxide, chlorates, nitrates, etc.).
The combustion process flows the more intense than the more specific area of \u200b\u200bthe contact of the combustible substance with the oxidizing agent (paper trimming is intense than packs of paper) and the higher the concentration of the oxidant, temperature and pressure. If you eliminate at least one of the reasons that cause combustion, the process stops.
In case of fire, the temperature reaches 1000-1300s, and in some cases, for example, when combustion of magnesium alloys, - 3000c.
Explosion, detonation, outbreak, fire, self-burning, ignition, self-ignition - all this kind of burning.
Explosion - Extremely rapid chemical transformation, accompanied by excretion of energy and the formation of compressed gases capable of producing mechanical work. This work is carried out as a result of the occurrence of a shock wave - a hopping pressure of a pressure spreading in a medium with supersonic speed.
The spread of the explosion due to the passage of the shock wave by substance and flowing for this substance under these conditions with a constant supersonic speed (about thousands of thousand meters per second) is called detonation.
Under production conditions, explosive mixtures of combustible gases and vapors can form (at a certain concentration of them in the air) - gasoline, toluene, ethyl alcohol, acetone, ethyl acetate, etc. - in the departments of deep and flexographic printing, lacquer compartments, compartments of photopolymer forms, charging batteries. This can occur in the absence of effective system Ventilation, violation of technology, inconsistency of electrical installations requirements of PUE, etc. Explosive mixtures with air forms also in suspended dust of dust starch, paper, aluminum, magnesium, rosin, shellac, etc. The most dangerous dust, which forms explosive mixtures with
air at a concentration of up to 15 (aluminum, rosin, shellac, etc.).
Flash - Fast combustion of a combustible mixture, not accompanied by the formation of compressed gases. This distinguishes not enough heat for the formation of a new concentration of a combustible mixture vapor, and burning stops.
Ignition- The occurrence of burning under the action of the ignition source.
Spontaneous combustion - the phenomenon of a sharp increase in the speed of exothermic reactions, leading to the occurrence of combustion of the substance (material, mixture) in the absence of a ignition source. Self-burning can be thermal, microbiological and chemical.
Thermal self-burning occurs with the external heating of the substance (material, mixture), exceeding the temperature of its self-burning, i.e. The lowest temperature at which it occurs is self-heating. For example, oak, pine, spruce wood and items from it at ambient temperature are more than 100 ° C well - there is a decomposition of its unstable connections. At 230-270c, the decomposition accelerates, and the oxidation begins. The process of decomposition of wood is exothermic, and if the heat released during oxidation exceeds the heat transfer to the environment, the accumulation of heat leads to self-burning.
To prevent thermal self-burning, it is necessary to prevent combustible substances and materials from the action of external heat sources.
Microbiological self-burning occurs as a result of self-heating arising under the influence of the life of microorganisms in the mass of matter (material, mixture). A substance of plant origin is inclined to microbiological self-burning (mainly not dried) - hay, straw, sawdust, leaves, wet loose peat, etc.
Chemical self-burning arises as a result of chemical interaction of substances. For example, some brown and stone coals, folded in the boils, are capable of self-sucking and adsorption and in case of insufficient heat transfer to the environment - to self-turn. If you wash fibrous or crushed materials (for example, wool, rags, wood or even metal sawdust) with vegetable oils or animal fats, then they are distributed by a thin layer along the large surface of these materials, and then intensively oxidized and polymerized, which is accompanied by significant heat release. The wool fibrous material, folded in the pile, has low heat transfer into the environment. Therefore, the accumulated heat contributes to the acceleration of the oxidation process and polymerization, as well as a further increase in temperature. As soon as the temperature of the washed material reaches the oil ignition temperature, its self-burning will occur.
Mineral oils (oil refining products) are not inclined to self-burning.
Ignition - It is a fire, accompanied by the appearance of a flame.
Self-ignion - Self-burning, accompanied by the appearance of flame.
In practice industrial enterprises There are cases of self-burning of woolly vertier materials and workwear folded into the pile; Locches, the coating layer of which contains linseed oil.
Some chemicals can be self-turn or cause the fire of other substances in the air, with the action of water on them and when mixed with each other.
As a result of the oxidation reaction, especially in the presence of moisture, some metal powders (aluminum and zinc) are sparkled,
therefore, they must be stored in hermetically closed vessels.
To substances causing burning under the action on them of water include calcium carbides and alkali metals, alkaline and alkaline earth-earth hydrides, etc. These substances, when interacting with water, usually distinguish combustible gases, which, heating due to the heat of the reaction, are self-turn.
To substances that self-turning when mixed with each other include chlorine and other halides, nitric acid, chrome anhydride, chlorine lime, sodium peroxide and potassium, etc. Some of these oxidizers when mixed or contacting at normal temperature with organic substances can cause their self-burning. . Other self-turn under action on a mixture of oxidizing agent with combustible substance, sulfur or nitric acids, when heated or heated.
It includes phosphorus, zinc and aluminum dust, sulfides, alkali metal carbides, etc.
The inclination to the self-burning of substances and materials is taken into account when developing measures fire prevention When they are stored, transportation, drying, performing technological operations, etc.
The list of indicators necessary to assess the fire hazard and fire hazard of substances and materials, depending on their aggregate state, is given in Table. 1 Annexes to federal law "Technical Regulations on Requirements fire safety. the federal law RF 123. "
The main indicators when evaluating the fire hazard of liquids are: a combustibility group; Flash temperature; Flammation temperature and concentration limits of ignition. The main indicators in assessing the fire hazard of solids and materials - a combustibility group; Flammation temperature, self-ignition temperature, tendency to self-burning.
A combustibility group. Substances and materials are divided into combustion into three groups: non-combustible, i.e. incapable of conventional combustion in the air; Possible, which can be flashed and burn in the presence of a source of ignition, but are not able to burn independently when it is removed; Combustible, sparkling from the ignition source and continue to burn when it is removed. Combustible materials are divided, in turn, on flammable, i.e. Such that ignite from the ignition source of minor energy (match, spark, etc.) without prior heating, and faintless, which are ignited only from a relatively powerful ignition source.
Flash temperature is the lowest (under special test conditions) The temperature of a combustible substance, in which pairs and gases capable of flashing in the air from the ignition source are formed above its surface, but the speed of their education is still insufficient for subsequent burning.
The term "flash temperature" usually refers to flammable liquids, but some solids (camphor, naphthalene, phosphorus, etc.), evaporate at normal temperature, are also characterized by a flash point. The lower the flammable temperature of the fuel fluid, the greater danger represents it in the fireplace.
According to the rule of Ormandi and Gravsen, the outbreak temperature is equal to
t B \u003d T kip. X K.
where is the boiling point, hail. TO; K is a coefficient equal to 0.736.
Fire hazard depending on the outflow temperature, combustible fluids are divided into two classes:
1st grade - flammable liquids (LVZ) - gasoline, toluene, benzene, acetone, methyl and ethyl alcohol, ether, kerosene, turpentine, etc.;
2nd grade - combustible fluids (GJ) - mineral oils, fuel oil, formalin et al.;
The ignition temperature is the temperature of a combustible substance at which it highlights combustible pairs and gases at such a speed that after igniting them from the ignition source there is a steady burning.
The self-ignition temperature is the lowest temperature of the substance (material, mixtures), in which the rate of exothermic reactions ending with the burning to the formation of the flame increases dramatically.
The temperature of self-ignition is not constant even for the same substance. It depends on the concentration of oxygen in the air, pressure, heat transfer conditions in the environment, etc. For example, the self-ignition temperature of combustible gases and vapors ranges in the range of 300-700s, wood, peat, paper, cardboard - 250-400C, celluloid - 140-180s, viniplast - 580s, rubber - 400c.
The concentration limits of ignition are the minimum and maximum concentration of the flammability region, i.e. The concentrations of the combustible substance, inside of which its mixtures with this oxidizing agent (usually air) are capable of flammable from the ignition source, followed by the propagation of burning along the mixture, however far from the ignition source. For example, for acetone, the lower concentration limit of ignition (explosion) is 2.6%, and the upper - 12.2% (volume), for gasoline A-76, respectively, 0.76% and 5.03%, for ethyl alcohol - 3, 3% and 18.4%, natural Gas. 5% and 16%, etc.
The explosion of combustible gases, vapors and dust is the greater, the smaller the lower concentration limit of ignition and the greater the gap between the lower and the upper limits of ignition. Thus, the explosion is directly proportional to the size of the flammable area.
Fires are classified by the type of combustible material and are divided into the following classes.
Fires of solid combustible substances and materials (s).
Fires combustible fluids or melting solids and
materials (B).
Fires gases (c).
Fires metals (D).
Fires of combustible substances and materials of electrical installations under stress (E).
Fires of nuclear materials, radioactive waste and radioactive substances (F).
 Indicators of fire hazard substances.To complete the fire hazard assessment of solids and materials, as well as liquids and gases, certain indicators are needed.
Indicators of fire hazard substances.To complete the fire hazard assessment of solids and materials, as well as liquids and gases, certain indicators are needed.
Temperature ignitionthe smallest temperature of the combustible substance is called, at which it highlights combustible pairs or gases at such a speed, which after igniting them from an external ignition source, the substance is stable. Inflammation temperature is an indicator of fire hazard only combustible substances and materials, since it characterizes their ability to self-burning.
Self-flamement temperature It is called the smallest temperature of the substance (or its mixture with air), at which there is a sharp increase in the speed of exothermic reactions, leading to the occurrence of flame burning.
The ignition temperature of gases and vapors take into account in cases:
classifications of gases and vapors of flammable liquids for explosion hazard groups to select the type of electrical equipment (while meaning the standard self-ignition temperature);
select the temperature conditions of the safe use of the substance when he is heated to high temperatures (while use the minimum temperature of self-ignition);
calculations of the maximum permissible heating temperature of non-insulated surfaces of technological, electrical and other equipment;
investigation of the causes of the fire, if it is necessary to determine if the substance from the heated surface could be self-splashing.
Template to self-burningit characterizes the ability of a number of substances and materials to be self-turn when heated to relatively small temperatures or contact with other substances, as well as when exposed to heat released by microorganisms in the process of their livelihoods. In accordance with this, thermal, chemical and microbiological self-burning distinguish.
Tendency to thermal self-burning It is characterized by self-heating temperatures and tensions, as well as the dependence of the temperature of the medium in which the self-burning is observed, from the size and shape of the sample. The tendency to self-burning is taken into account in the development of fire and preventive measures.
Self-heating temperature The smallest temperature is called in substance or material, almost distinguishable exothermic oxidation and decomposition processes arise, which can lead to self-burning.
Heating to self-heating temperature - the smallest temperature of the substance, can potentially represent fire hazard. The self-heating temperature is taken into account when determining the conditions for the safe long (or permanent) heating of the substance.
Safe heating temperature This substance or material (regardless of the sample size) should be considered a temperature not exceeding 90% of the temperature of self-heating.
Temperature drainagethe critical temperature of the solid is called, in which the rate of self-heating process dramatically increases, which leads to the occurrence of the focus. Treatment temperature takes into account when investigating the causes of fires, determining safe Conditions Heating solid materials, etc.
Consider the peculiarities of the process of oxidation of self-turning substances of plant origin, fossil coal, oil and fat, chemicals and mixtures.
Among the self-turning substances of plant origin include Meal, fish flour, hay, cake, etc. Especially susceptible to self-burning wet vegetable products in which the life of microorganisms continues.
The presence of moisture in plant products at certain temperatures is accompanied by the reproduction of microorganisms, the intensification of the vital activity of which causes an increase in temperature. Vegetable products are bad heat conductors, therefore they have a further increase in temperature.
With the conditions favorable to accumulate conditions: a significant mass of the plant product, for example, hay or cake in the hold, the temperature can reach 70 ° C.
At this temperature, microorganisms are dying, and their decomposition is accompanied by a further increase in temperature with the formation of porous coal, which is capable of absorbing pairs and gases in a large volume.
This process is also accompanied by heat release and gradual increase in temperature to 100 - 130 ° C, in which the decay of new compounds with the formation of porous coal. At a temperature of 200 ° C, the fiber is decomposed, which is part of the plant products, and forms the new kind Coal capable of intensively oxidized. The process of coal oxidation leads to a further increase in temperature, up to the occurrence of burning.
Coal obtained by thermal decomposition of cellulosic materials, such as charcoal, is capable of self-turn. And this happens immediately after its manufacture. Over time, its ability to absorb pairs and gases is reduced, as a result of which charcoal, which is in air for a long time, loses a tendency to self-burning.
Fossil coal of certain types is able to oxidize low temperatures and absorb oxygen from air and other gases or pairs. But the main reason Self-burning is the oxidation of coal. The absorption of vapor and gases with carbon is also accompanied by an increase in temperature.
The greatest absorption capacity has a young coal containing moisture. Thus, freshly brown coal contains 10 - 20% of hygroscopic moisture, and the skinny - approximately 1%, so the latter is more resistant to self-burning. The increase in moisture causes an increase in the temperature of the coal to 60 to 75 ° C, and the further heat release occurs due to the oxidation of the organic mass.
Development of the fossil coal self-burning process It depends on the degree of its shreddance: the smaller the coal, the greater the surface of the absorption and oxidation, the speed of their flow, the greater the heat is released.
Often the reason for the fire is self-burning of fats and oils of mineral, vegetable or animal originwhich are impregnated with fibrous materials and fabrics.
Mineral oils (machine, solarium, transformer) are a mixture of limit hydrocarbons and cannot be self-turn in pure form. Self-burning them is possible in the presence of impurities of vegetable oils. Vegetable oils (hemp, linen, sunflower, cotton) and animal oil (butter) are a mixture of fatty acid glycerides.
Many chemicals and mixtures of mixtures with air or moisture are capable of self-escaping. These processes often end with self-burning.
By self-burning ability, chemicals are divided into three groups:
1st group.
Substances, self-turning in touch with air(activated carbon, phosphorus white, vegetable oils and fats, sulfur metals, aluminum powder, alkali metal carbide, powder-shaped iron, zinc, etc.).
The oxidation of some substances of this group caused by their interaction with water pairs of air is accompanied by the release of a large amount of heat and flows as fast as it turns into burning or an explosion. For other substances, self-heating processes continue for a long time (for example, the process of self-burning of white phosphorus ends with a burning after a few seconds, and the process of self-combustion of freshly prepared activated coal lasts for several days).
2nd group.
Corustion substances when interacting with water(Alkaline metals and their carbides, calcium oxide (smooth lime), sodium peroxide, phosphorous calcium, phosphorous sodium, etc.).
The interaction of alkali metal with water or water moisture is accompanied by the release of hydrogen, which is flammable due to the heat of the reaction. Hitting negamen lime A small amount of water causes self-heating ending with a strong heating (to the glow), therefore, the combustible materials that are nearby can be protected.
3rd group.
Substances, self-coating when mixing one with another. So, the effects of nitric acid on wood, paper, fabric, turpentine and essential oils causes inflammation of the latter; Chrome anhydride flammifies alcohols, ethers and organic acids; acetylene, hydrogen, methane and ethylene are self-turn in the atmosphere of chlorine in daylight; Grinding iron (sawdust) is self-turning in the chlorine atmosphere; Alkali metal carbides are flammored in the chlorine atmosphere and carbon dioxide.
Temperature Flash The smallest temperature of the combustible substance is called, in which under the conditions of special tests over its surface, pairs or gases are formed, capable of flashing in the air from an external ignition source.
Flash temperature is a parameter that indicates temperature conditionsin which a fuel becomes flammable. The flashing temperature of flammable liquids under this classification is determined only in the closed crucible.
Inflammation areagases (vapor) in the air is called the region of the concentration of this gas in the air when atmospheric pressure, inside which gas mixtures with air are capable of flammable from an external ignition source, followed by the spread of the flame in the mixture.
The boundary concentrations of the region of ignition are called respectively lower and upper limits of ignition Gas (vapor) in the air. The values \u200b\u200bof ignition limits are used in calculating the permissible concentrations of gases inside the explosive technological devices, ventilation systems, as well as when determining the maximum permissible explosive concentration of vapors and gases during operations with the use of fire sparkling.
The magnitude of the gas concentration or steam in the air inside the technological apparatus not exceeding the 50% magnitude of the lower limit of ignition, can be taken as explosionable concentration. Ensuring explosion safety Environments inside the equipment under normal technological mode does not give reason to consider this equipment unbreakable.
For the magnitude of the maximum permissible explosion-proof concentration (PDV) of vapors and gases when working with fire, sparkling tool should be taken to be concentrated, which does not exceed 5% of the lower limit of the inflammation of this steam or gas in the air in the absence in the condensed phase under consideration.
Temperature limits of ignition of vapors in the airthe temperature boundaries of the substance under which saturated couple form concentrations equal to the bottom or top concentration limit ignition.
Temperature limits of ignition are taken into account when calculating safe temperature modes in closed technological volumes with liquids (fuel freight tanks, etc.), working at atmospheric pressure.
Safe, regarding the possibility of the formation of explosive steadmate mixtures, the temperature and maximum explosion pressure should be considered.
Maximum explosion pressure - This is the greatest pressure resulting from the explosion. It is taken into account when calculating the explosion-resistant equipment with flammable gas, liquids and powdered substances, as well as safety valves and explosive membranes, the shells of blasting electrical equipment.
Furniture indicator (coefficient K) ~the dimensionless value expresses the ratio of the amount of heat released by the sample in the process of testing to the amount of heat generated by the ignition source,
where q. - heat isolated by the sample in the process of burning, kcal;
q I. - thermal impetus, i.e. Heat supplied to the sample from a permanent source
ignition, kcal.
According to the results of the test, the degree of ignition is assessed as follows.
Materials non-burning- Materials that, when heated to 750 ° C, are not lit and in air do not emit combustible gases in an amount sufficient to ignite them from the fallen flame. Since determined by the method of calorimetry coefficient TO< 0.1, such materials are not able to burn in air.
Materials of employment- materials, the ignition temperature of which is lower than 750 ° C, and the material is on, smolders or coats only under the influence of the fallen flame and stops burning or smoldering after it is removed (0.1< TO< 0,5).
Materials are hardly ignorant (or self-tapping) - materials, the temperature of the ignition of which is lower than 750 ° C, and the material is on, smolders or coats under the influence of the fallen flame. After its removal, the material continues to burn with a fading flame, not racing sample (0.5< TO< 2,1). Такие материалы не способны возгораться в воздушной среде даже при длительном воздействии источника зажигания незначительной энергии (пламени спички 750 - 800°С, тления папиросы 700 - 750°С и т.д.).
Materials combustible - materials, the ignition temperature of which is lower than 750 ° C, and the material, igniting the fallen flame, continues to burn or smoke after its removal (TO> 2,1).
The speed of burning. The combustion rate of the solid depends on its shape. Grinding solids in the form of sawdust or chips will burn faster than monolithic. In the chopped fuel big surface The combustion is exposed to heat, so heat is absorbed far faster, evaporation occurs significantly more active, with the allocation of more vapors. The burning proceeds very intensively, as a result of which a furyed substance is spent quickly. On the other hand, the monolithic fuel will burn longer than crushed.
Dust clouds consist of very small particles. When the cloud of flammable dust (for example, grain) is well mixed with air and flammifies, the burning occurs very quickly and is often accompanied by an explosion. Such explosions were observed during loading and unloading grain and other crushed combustible substances.
There are two combustion speeds: mass and linear.
Most combustion rate There is a mass (t, kg) of a substance that burned out per unit of time (min, h).
Linear combustion rate of solid combustible substancesit is called the speed of propagation of fire (m / min) and the growth rate of the fire focus (m 2 / min). The combustion rate of solids depends on the degree of their grinding, humidity, bulk weight, air access and a number of other factors.
Study of fire cases on ships makes it possible to adopt the following average linear combustion rate (m / min) of various objects:
Posts of control ................................................ ..................... 0,5
Living spaces................................................ ................... 1.0-1,2
Economic premises, storage room combustible materials ..... 0.6-1.0
Freight facilities ..................................... ............. .............. 0.5-0.7
Decks of car ferries ............... ............................... 1 ,five
Machine compartment with DVS when burning diesel fuel under the plates .... 10
Separation of auxiliary mechanisms ......... ......................... 1,2
Electrical places ............................................. 0.8
Boiler houses when burning fuel oil under the plates ............. 8.0
Approximately during the first 2-3 minutes of the fire rapidly increases the area of \u200b\u200bits focus (on passenger ships to 20 m 2 / min). This time is usually taken to collect the vessel crew alarm and therefore active struggle With fire is not yet conducted. In the next 10 minutes, when stationary means of water and foaming, the growth of the fire focus is slowed down.
The linear speed of the spread of fire determines the area of \u200b\u200bthe fire, and the degree of burnout in everything that can burn on this area is the duration of the fire.
Linear fluid burning speedit is characterized by the height of its layer (mm, cm), burned out per unit of time (min, h). The rate of flame propagation when flamming combustible gases is from 0.35 to 1.0 m / s.
Speed \u200b\u200bburnoutit is characterized by the amount of fuel, burning per unit of time from a unit of burning area. It determines the intensity of the combustion of materials during the fire. It needs to be known to calculate the duration of the fire in any fluids. Fluid burnout speed, spilled on the surface sea water, approximately the same as when burning it from open surfaces tanks.
Temperature. The most important parameter of the ship fire, which is largely determining not only engineering and preventive measures, but also the tactical actions of emergency parties and groups of courts is the temperature. Special great importance It has a temperature at internal ship fires.
The intensity of heat transfer from the fire area into the environment, the speed of movement of gas flows, as well as the possibility of explosions representing extreme danger during fire extinguishing, depends on the fire temperature.
The temperature field of the fire is extremely inhomogeneously.The closer to the fire zone, the temperature is usually higher. At the top of the premises, the air is usually more heated than the decks. Taking into account the behavior of ship structures and materials and from a fire-tactical point of view, it is more convenient for the temperature of the fire to adopt the average temperature of flue gases that fill the fire zone. The temperature on the surfaces of the ship structures enclosing the fire zone are also essential: the temperature on the surface facing the fire and the temperature on the opposite surface of the surface.
Approximately the temperature at some points of the fire zone can be determined indirectly - on the melting of unburned materials, which were in the fire zone, or the color of the heated bodies (Table 4.1).
Table 4.1.
Dependence Color Caution from Temperature
When burning solid materialsthe fire temperature depends mainly on the kind of materials, the magnitude of the fire load, the conditions of air flow and the removal of combustion products, as well as the duration of burning.
The dependence of the fire temperature on the duration of burning for all solids has approximately the same character. Initially, the temperature increases sharply to the maximum, and as the material burns out, its gradual decline occurs. When the fire load increases, the total duration of burning increases, the maximum fire temperature increases, the temperature decline is slower, but the character of dependence remains unchanged.
In conditions of limited gas exchange, for example, with closed openings in a residential room, the increase in temperatures occurs significantly slower. The maximum temperature reaches 800 -900 ° C.
Temperature regime in rooms when burning liquids has its own characteristics. Since fluids are usually in any vessels (in pallets, tanks, etc.), their burning is often local in nature. Under these conditions, if the ratio of burning area to the deck square is close to one, the fire temperature is approximately 1100 ° C. If the combustion area is only a small portion of the deck area, the temperature is significantly lower.
Fire temperature with simultaneous combustion of liquids and solid materials It depends on which combustible materials prevail: if liquids are only a small part of the fire load, then temperature mode It differs little from the mode of solid materials.
With internal fires in the zone of aggressive exposure to heat may be sudden convective flows Hot gases that arise when changing the conditions of gas exchange caused by opening doors and other openings.
The zone of aggressive heat exposure is part of the smoke zoneIt may have dangerous temperatures for man. A person is capable of a very short time to be in a dry air having a temperature of 80 - 100 ° C. Long stay at a temperature of 50 - 60 ° C causes the greatest consequences of overheating. Wet air at a temperature of 50 - 60 ° C for many people becomes intolerable in a few minutes.
When evaluating the fire hazard of gases Determine the area of \u200b\u200binflammation in the air, the maximum explosion pressure, the temperature of self-ignition, the category of explosive mixture, minimal ignition energy, minimal explosive oxygen content, nominal speed burning.
When evaluating fire hazard liquidsdetermine the combustibility group, flash temperature, flammability temperature, temperature limits of ignition, burnout speed. For flammable liquids, the area of \u200b\u200binflammation in the air is additionally determined, the maximum explosion pressure, the explosive mixture category, minimal ignition energy, minimal explosive oxygen content, normal burning speed.
When evaluating fire hazard All solids and materials are determined by a group of ignition, ignition temperature. For solids with a melting point below 300 ° C, additionally determine: flash temperature, temperature limits of vapor flames in the air.
For porous, fibrous and bulk materials, if necessary, additionally determine the temperature of the self-heating, the temperature of the tension during self-burning, the temperature conditions of thermal self-burning.
For substances, powdered or capable dust are additionally determined by the lower limit of the inflammation of the aircraft, the maximum pressure of the explosion of the aircraft, the minimum energy of the airproof, the minimum explosive oxygen content.
When evaluating the fire hazard of the substance It is necessary to study its properties, identify the possibility of changing them over time and when used under certain conditions. In particular, it is important to consider in contact with the substance with other active substances with prolonged heating, irradiation and other external influences, as a result of which its physicochemical properties may change.
When testing shipbuilding, as well as other solid materials for ignoram, the group of combustible materials is initially detected method of fire pipe.
The material is considered combustionIf, when tested by a fire pipe, the time of self-combustion or tension exceeds 1 min, and the weight loss of the sample is 20%. Materials, independently burning flames along the entire surface of the sample, are also among the entire surface of the sample, regardless of the weight loss and the time of its burning. Such materials are not subjected to further tests.
Materials having weight loss are less than 20%, as well as materials that lose 20% of weight and more, but independently burning or smaller than 1 min for the final evaluation of the degree of ignition are subject to additional tests for the method of calorimetry.