Conditions required for combustion. Combustion conditions. What to do in case of a fire in the forest
It is known that for combustion to occur, it is necessary to have:
1. Combustible substance
2. Oxidant
3. Ignition source (energy pulse)
These three components are often referred to as the fire triangle. If one of them is excluded, then combustion cannot occur. it essential property The triangle is used in practice to prevent and extinguish fires.
Air and combustible matter make up a system capable of burning, and temperature conditions cause the possibility of self-ignition and combustion of the system.
The highest rate of combustion is obtained when the substance is burned in pure oxygen, the lowest (cessation of combustion) - when the content of oxygen is 14–15%.
Combustion of substances can occur due to oxygen, which is in the composition of other substances that can easily give it away. Such substances are called oxidizing agents. Here are the most famous oxidants.
· Berthollet's salt (KClO 3).
Potassium nitrate (KNO 3).
· Sodium nitrate (NaNO 3).
Oxidants contain oxygen, which can be released by decomposition of salt, for example:
2 KClO 3 = 2KCl + 3 O 2
Decomposition of oxidants occurs when heated, and some of them even under the influence of a strong shock.
2. Combustion products. Complete and incomplete combustion. Environmental aspects of combustion processes.
Combustion products are formed during combustion. The composition of us depends on the burning substance and combustion conditions. Combustion products, with the exception of carbon monoxide, are incapable of burning.
The smoke generated during the combustion of organic substances contains solid particles and gaseous products (carbon dioxide, carbon monoxide, nitrogen, sulfur dioxide, and others). Depending on the composition of substances and the conditions of their combustion, smoke of different content is obtained. The fumes formed during the combustion of various substances differ not only in composition, but in color and smell. The color of the smoke indicates which substance is burning, although the color of the smoke changes depending on the friction conditions. When wood burns, the smoke has a grayish-black kick; paper, hay, straw - whitish yellow; fabric and cotton - brown; petroleum products - black, etc.
Combustion products are gaseous, liquid or solids formed during combustion. The composition of combustion products depends on the composition of the burning substance and on the conditions of its combustion. Organic and inorganic fuels are mainly composed of carbon, oxygen, hydrogen, sulfur, phosphorus and nitrogen. Of these, carbon, hydrogen, sulfur and phosphorus are capable of oxidizing at the combustion temperature and forming combustion products: CO, CO 2, SO 2, P 2 O 5. Nitrogen at the combustion temperature is not oxidized and is released in a free state, and oxygen is consumed for the oxidation of the combustible elements of the substance. All these combustion products (with the exception of carbon monoxide CO) are no longer capable of burning in the future. They are formed during complete combustion, that is, during combustion, which occurs when a sufficient amount of air is available and at high temperatures.
Carbon dioxide or carbon dioxide (CO 2) - a product of complete combustion of carbon. Odorless and colorless. The combustion of magnesium, for example, occurs in an atmosphere of carbon dioxide according to the equation:
CO 2 +2 Mg = C + 2 MgO .
If the concentration of carbon dioxide in the air exceeds 3-4.5%, being in the room and inhaling the gas for half an hour is life-threatening.
Carbon monoxide or carbon monoxide (CO) - a product of incomplete combustion of carbon. This gas is odorless and colorless, therefore it is especially dangerous.
Sulphur dioxide(SO 2) is a combustion product of sulfur and sulfur compounds. Colorless gas with a characteristic pungent odor.
Smoke During the combustion of many substances, in addition to the combustion products discussed above, smoke is released - a dispersed system consisting of the smallest solid particles suspended in any gas.
Incomplete combustion of organic matter at low temperatures and lack of air produces more diverse products - carbon monoxide, alcohols, ketones, aldehydes, acids and other complex chemical compounds. They are obtained by partial oxidation of both the fuel itself and the products of its dry distillation (pyrolysis). These products produce pungent and toxic fumes. In addition, the products of incomplete combustion are themselves capable of burning and forming explosive mixtures with air. Such explosions occur when extinguishing fires in basements, dryers and in closed rooms with a large amount of combustible material. Let us consider briefly the properties of the main combustion products.
Environmental aspects of combustion processes. Application natural gas allows to reduce air pollution with sulfur oxides, particulate matter and carbon monoxide, however, a large amount of nitrogen oxides, carbon monoxide and carcinogenic substances (3,4-benz (o) trans) enter the atmosphere. Correct organization of combustion, selection of rational combustion methods allows minimizing the formation of harmful substances and their release into the air basin. The use of natural gas makes it possible to conduct not only passive, but also an active struggle for air purity: the use of afterburner installations, the use of exhaust gases for supplying gas burner instead of the corresponding amount of air.
Ecological problems of combustion. The task is not to harm when burning fuels. Negative manifestations:
Man-made heat release is commensurate with the components heat balance atmosphere;
The acoustic noise of turbulent flames during the operation of aircraft and rocket engines is an environmental pollutant.
The emission of harmful combustion products - nitrogen oxides, metal oxides, carbon monoxide (at high Tg), sulfur oxides, carcinogenic substances - products of incomplete pyrolysis of organic fuels, soot, carbon dioxide (at low Tg) - is the reason: changes in the optical properties of the atmosphere and a decrease in solar radiation flux, acid rain, increased "greenhouse effect", destruction of the ozone layer of the Earth, negative impact on flora and fauna, buildings and structures. Overall result: global warming, climatic disasters (cyclones, storms, tornadoes, tsunamis, floods, droughts, avalanches, mudflows) ..
3. Equations of combustion of substances in oxygen and air, the method of their compilation. Combustion thermodynamics. Heat effects of combustion reactions.
The general equation of the reaction of combustion of any hydrocarbon
C m H n + (m + n / 4) O 2 = mCO 2 + (n / 2) H 2 O + Q (8.1)
where m, n is the number of carbon and hydrogen atoms in the molecule; Q is the heat of reaction, or heat of combustion.
Thermal effect (heat of combustion) Q - the amount of heat released during the complete combustion of 1 kmol, 1 kg or 1 m 3 of gas under normal physical conditions. Distinguish between the highest Q in and the lowest Q n heat of combustion: the highest heat of combustion includes the heat of condensation of water vapor during combustion (in reality, when gas is burned, water vapor does not condense, but is removed along with other combustion products). Typically, technical calculations are usually based on the lowest heat of combustion, excluding the heat of condensation of water vapor (about 2400 kJ / kg).
The efficiency, calculated from the lowest heat of combustion, is formally higher, but the heat of condensation of water vapor is high enough, and its use is more than expedient. This is confirmed by the active use of contact heat exchangers in heating technology, which are very diverse in design.
For a mixture of combustible gases, the highest (and lowest) heat of combustion of gases is determined by the ratio
Q = r 1 Q 1 + r 2 Q 2 + ... + r n Q n (8.2)
where r 1, r 2, ..., r n - volumetric (molar, mass) fractions of the components included in the mixture; Q 1, Q 2,…, Q n is the heat of combustion of the components.
The combustion process is much more complicated than according to formula (8.1), since along with the branching of the chains, they are terminated due to the formation of intermediate stable compounds, which undergo further transformations at high temperatures. With a sufficient concentration of oxygen, the final products are formed: water vapor H 2 O and carbon dioxide CO 2. With a lack of an oxidizing agent, as well as with cooling of the reaction zone, intermediate compounds can stabilize and get into environment.
High-temperature combustion of hydrocarbons is very complex and is associated with the formation of active particles in the form of atoms and radicals, as well as intermediate molecular compounds. As an example, the reactions of combustion of the simplest hydrocarbon, methane, are given:
1.
H + O 2 - ›OH + O
CH 4 + OH -> CH 3 + H 2 O
CH 4 + O - ›CH 2 + H 2 O
2.
СН 3 + О 2 - ›НСНО + ОН
СН 2 + О 2 - ›НСНО + О
3.
НСНО + ОН - ›НСО + Н 2 О
НСНО + О - ›СО + Н 2 О
НСО + О 2 - ›СО + О + ОН
4.
CO + O - ›CO 2
CO + OH - ›CO 2 + H
Single cycle result:
2СН 4 + 4О 2 - ›2СО 2 + 4Н 2 О
Combustion thermodynamics
The initial composition of the combustible mixture is characterized by the molar or mass fractions of the components and the initial pressure and temperature. If the composition of the mixture is selected so that during its combustion both the fuel and the oxidant are completely converted into reaction products, then such a mixture is called stoichiometric. Excessive fuel mixtures are called rich, and with a lack of fuel - poor... The degree of deviation of the mixture composition from stoichiometric is characterized by the excess fuel ratio (rus. equivalenceratio) :
where Y F and Y O- mass fractions of fuel and oxidizer, respectively, and (Y F/Y O) st- their ratio in the stoichiometric mixture. In the Russian-language literature, the oxidizer (or air) excess ratio is also used, which is the reciprocal of the fuel excess ratio.
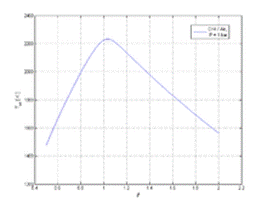
Adiabatic combustion temperature of CH 4 mixtures with air, depending on the fuel excess ratio. P = 1 bar, T 0 = 298.15 K.
If combustion occurs adiabatically at a constant volume, then the total internal energy of the system is conserved; if at constant pressure, then the enthalpy of the system. In practice, the conditions of adiabatic combustion are approximately realized in a freely spreading flame (without taking into account heat loss by radiation) and in other cases when heat losses from the reaction zone can be neglected, for example, in combustion chambers of powerful gas turbine plants or rocket engines.
The adiabatic combustion temperature is the temperature of the products reached when chemical reactions are complete and thermodynamic equilibrium is established. For thermodynamic calculations, tables of thermodynamic functions of all components of the initial mixture and products are used. Methods of chemical thermodynamics allow calculating the composition of products, final pressure and temperature under given combustion conditions. There are many programs currently available that are capable of performing these calculations.
The heat of combustion is the amount of heat released during the complete combustion of the original components, that is, up to CO 2 and H 2 O for hydrocarbon fuels. In practice, part of the released energy is spent on the dissociation of the products; therefore, the adiabatic combustion temperature, disregarding dissociation, turns out to be noticeably higher than that observed in practice.
Thermodynamic calculation allows one to determine the equilibrium composition and temperature of products, but does not give any information about how fast the system is approaching the equilibrium state. Full description combustion requires knowledge of the mechanism and kinetics of reactions and conditions of heat and mass transfer with the environment.
4. Flame types and burning rate. Combustion theories: heat, chain, diffusion.
In the general case, the combustion rate depends on the mixing rate of the initial components in the heating zone and the reaction zone (for heterogeneous systems), on the rate of chemical reactions between the components, on the rate of transfer of heat and active particles from the reaction zone to the initial system. The normal combustion rate (and even more so the shape of the combustion front) depends on the flow conditions of the fresh mixture and combustion products (especially when burning in engines).
Therefore, in the theory of combustion, several basic types of flames are considered. They are not the same in their scientific and practical significance and degree of study. The parameters of greatest interest for of this type flame. The approach to theoretical consideration of each type of flame is significantly different. There are some differences in experimental methods as well.
We list the most important types of flame for the theory of combustion:
1) laminar flame in a homogeneous gas mixture. Flames from volatile explosives are of the same type;
2) laminar diffusion flame during combustion of a jet of combustible gas in an oxidizing atmosphere. A flame adjoins this type during diffusion combustion of liquid fuel poured into cylindrical vessel, etc.;
3) flame during combustion of a drop of liquid fuel or a particle of solid fuel in an oxidizing atmosphere;
4) turbulent flames in homogeneous or in premixed gas mixtures;
5) flame during combustion of non-volatile explosives, propellants, etc. in cases where the reaction in the condensed phase plays an essential role.
Let us briefly consider some of the characteristics of the main types of flames to the extent that it is useful for understanding the laws of combustion of condensed mixtures.
Preliminarily, one should dwell on the definition burning rate ... With laminar combustion gas mixtures and homogeneous condensed systems of great fundamental importance is the concept of the normal burning rate ( uн). By definition, uн is equal to the speed of movement of the flame relative to the fresh mixture in the direction perpendicular to the flame surface at a given point. Dimension uн in the SI system - m / s, however, for the burning rate, this unit is still rarely used and only for gas systems. Usually the value uн for gas systems, they are expressed in cm / s, and for condensed systems, in mm / s (if the combustion rate of condensed systems is expressed in m / s, then very small fractional numbers are obtained in the usual pressure range).
For homogeneous condensed systems, the combustion rate of cylindrical charges burning from the end is most often measured, and the combustion front is assumed to be flat (experience shows that in most cases, in the presence of a proper shell, this assumption is valid, and distortions are observed only at the edges of the charge). In addition, for solid substances (and sufficiently viscous liquid substances), the initial (solid or liquid) substance is motionless during combustion. Therefore, in this case, the normal combustion rate is simply equal to the apparent flame speed (in the laboratory coordinate system) and is constant at different points of the charge.
Combustion is called complex physical- chemical process the interaction of a combustible substance and an oxidizer, characterized by a self-accelerating chemical transformation and accompanied by the release a large number heat and light. Flame combustion can occur either under the influence of an ignition source (ignition), or as a result of a sharp increase in the rate of exothermic reactions (self-ignition).
Self-ignition mode consists in the spontaneous occurrence of flame combustion of a combustible mixture preheated to a certain critical temperature (the so-called autoignition temperature); this mode manifests itself in the form of a flash and is characterized by the simultaneous combustion of the entire combustible mixture. Table 1 lists some flammable substances and their autoignition temperatures.
Table 1.
Autoignition temperature of some combustible substances
Ignition mode represents the propagation of a combustion wave (propagation of a flame front) through a cold mixture when it is locally ignited (ignited) by an external source. A flame is the visible combustion zone in which glow and heat emission are observed. The resulting flame itself becomes a source of heat flux and reactive particles into the adjacent layers of the fresh combustible mixture, thereby ensuring the movement of the flame front.
About spontaneous combustion of plant products... From plant products hay, straw, leaves, malt, hops are prone to spontaneous combustion. Particularly susceptible to spontaneous combustion are under-dried plant products, in which vital activity continues. plant cells.
According to the bacterial theory, the presence of moisture and an increase in temperature due to the vital activity of plant cells promotes the multiplication of microorganisms present in plant products. As a consequence poor thermal conductivity of vegetable products, the released heat gradually accumulates and the temperature in the mass of the product rises. At elevated temperatures, microorganisms die and turn into porous coal, which has the property of heating up due to intense oxidation and therefore is the next source of heat generation after microorganisms. The temperature in plant products rises to 300 ° C, and they ignite spontaneously.
Wood, lignite and bituminous coal, peat also ignite spontaneously due to intensive oxidation with atmospheric oxygen.
Vegetable and animal fats, if applied to crushed or fibrous materials (rags, ropes, tow, matting, wool, sawdust, soot, etc.) have the ability to ignite spontaneously.
When crushed or fibrous materials are wetted with oil, it spreads over the surface and, in contact with air, begins to oxidize. Simultaneously with oxidation in the oil, a polymerization process occurs (combining several molecules into one). Both the first and the second processes are accompanied by a significant release of heat. If the generated heat is not dissipated, i.e. builds up inside a tightly packed bale, the temperature in the oily material rises and can reach autoignition temperature.
Combustion occurs when there are three essential components: combustible substance, oxidizer and ignition source. Let's dwell on each of them in more detail.
Under the term combustible substance a substance is meant that is capable of self-burning after the external ignition source has been removed. The combustible substance can be in a solid, liquid or gaseous state. Most organic substances, a number of gaseous inorganic compounds and substances, many metals, etc. are combustible substances. Gases pose the greatest fire and explosion hazard.
Combustion of liquid. For ignition flammable liquid above its surface, a vapor-air mixture must first form. Combustion of liquids is possible only in the vapor phase, while the surface of the liquid itself remains relatively cold. Among flammable liquids (FL), a class of the most dangerous representatives is distinguished - flammable liquids (FL). Flammable liquids include gasolines, acetone, benzene, toluene, some alcohols, ethers, etc.
There are a number of substances (gaseous, liquid or solid) that can spontaneously ignite on contact with air without preheating (at room temperature), such substances are called pyrophoric. These include: hydrogen fluoride, white phosphorus, hydrides and organometallic compounds of light metals, etc.
There is a fairly large group of substances, upon contact of which with water or water vapor in the air, a chemical reaction begins, proceeding with the release of a large amount of heat. Under the influence of the released heat, self-ignition of combustible reaction products and initial substances occurs. This group of substances includes alkali and alkaline earth metals (lithium, sodium, potassium, calcium, strontium, uranium, etc.), hydrides, carbides, phosphides of these metals, low molecular weight organometallic compounds (triethylaluminum, triisobutylaluminum, triethylboron), etc.
Combustion of solid occurs according to a more complex mechanism and several stages are inherent in it. When exposed to an external source, the surface layer of the solid is heated, and gaseous volatile products are released from it. This process can be accompanied either by the melting of the surface layer of the solid, or by its sublimation (the formation of gases, bypassing the melting stage). Upon reaching a certain concentration of combustible gases in the air (lower concentration limit), they ignite and, through the released heat, begin to act on the surface layer themselves, causing it to melt and new portions of combustible gases and solid vapors entering the combustion zone.
Consider wood as an example. When heated to 110 ° C, the wood dries up and the resin evaporates slightly. Weak decomposition begins at 130 ° C. More noticeable wood decomposition (color change) occurs at 150 ° C and above. The decomposition products formed at 150-200 ° C are mainly water and carbon dioxide, therefore they cannot burn. At temperatures above 200 ° C, the main component wood - fiber. The gases produced at these temperatures are flammable as they contain significant amounts of carbon monoxide, hydrogen, hydrocarbons and other organic vapors. When the concentration of these products in the air becomes sufficient, under certain conditions they will ignite.
If a combustible substance melts while spreading, it increases the combustion site (for example, rubber, rubber, metals, etc.). In the event that the substance does not melt, oxygen gradually comes to the surface of the fuel and the process takes the form of heterogeneous combustion (the stage of burning out the coke of the carbon fuel). The combustion process of solids is complex and diverse, it depends on many factors (dispersion of a solid material, its moisture content, the presence of an oxide film on its surface and its strength, the presence of impurities, etc.).
More intensively (often with an explosion), fine-dispersed metal powders and dust-like combustible materials (for example, wood dust, powdered sugar) are ignited.
How oxidizing agent most often, during a fire, oxygen is released, the content of which in the air, as is known, is about 21%. Strong oxidizing agents are hydrogen peroxide, nitric and sulfuric acids, fluorine, bromine, chlorine and their gaseous compounds, chromic anhydride, potassium permanganate, chlorates and other compounds.
When interacting with metals, which exhibit very high activity in the molten state, water, carbon dioxide and other oxygen-containing compounds, which in common practice are considered inert, act as oxidants.
However, only the presence of a mixture of fuel and oxidizer is not enough to start the combustion process. Still needed ignition source... In order for a chemical reaction to occur, a sufficient number of active molecules, their fragments (radicals) or free atoms (which have not yet managed to combine into molecules), which have excess energy equal to or exceeding the activation energy for a given system, must appear.
The appearance of active atoms and molecules is possible when the entire system is heated, when gases are in local contact with a heated surface, when exposed to a flame, an electric discharge (spark or arc), local heating of the vessel wall as a result of friction or when a catalyst is introduced, etc. The source of ignition can also be sudden adiabatic (without heat exchange with the environment) compression. gas system or exposure to a shock wave.
Currently, scientists have established that the mechanism of the occurrence and development of real fires and explosions is characterized by a combined chain-thermal process. Having started in a chain way, the oxidation reaction, due to its exothermicity, continues to accelerate due to heat. Ultimately, the critical (limiting) conditions for the onset and development of combustion will be determined by the heat release and the conditions of heat and mass transfer of the reacting system with the environment.
Combustion is a chemical reaction (oxidation of a combustible substance), which is accompanied by the release of heat and light. To carry out combustion, you need: an oxidizing agent (for example, oxygen); source of ignition (combustible substance, e.g. wood); a source of flame (spark or heat release from chemical reactions, friction, short circuit). If one of the 3 conditions is absent, then combustion is not possible. A combustible substance can be a solid, liquid or gaseous substance, which under certain conditions can undergo chemical reactions with the release of heat. Combustible substances are divided into: rich mixtures in which there is an excess amount of combustible material than is required for combustion; lean mixtures - less combustible substance than is required for combustion, stoichiometric - at which the most complete combustion occurs. Therefore, rich and lean mixtures burn less intensely than stoichiometric ones.
Uncontrolled burning is a fire. A fourth is added to the three combustion conditions: the path of fire propagation. Incomplete combustion of substances produces smoke, which may contain toxic substances: carbon monoxide (carbon monoxide), vapors of acids, alcohols, aldehydes, etc. For example, when celluloid burns, hydrocyanic acid is formed. A fire is dangerous for a person due to lack of oxygen, explosions, destruction and panic.
> Types of combustion
1. Detonation. The substance instantly turns into a gaseous or dusty state. The burning speed is greater than the speed of sound. Pressure rises sharply.
2. Explosion - rapid combustion of combustible substances with the formation of a large amount of heat and gas capable of performing mechanical work. An explosion occurs in a gas or vapor-dusty environment. In this case, the temperature of this environment plays a secondary role. The main condition for an explosion is the presence of an appropriate concentration limit. The lower and upper flammable concentration limits in this case are already the lower and upper explosion limits (explosion limit). Second necessary condition for an explosion - the presence of a heat impulse of sufficient power. The explosion progresses like an avalanche. For a thermal explosion to occur, it is sufficient for the ignition source to heat up several molecules of the mixture. The heat generated from them will heat and ignite the nearest particles of the mixture. It should be noted that a gas or vapor-air mixture at the same concentrations can give a quiet stationary combustion or an almost instantaneous destructive explosion. Obviously, everything depends on the conditions under which the combustible substance is mixed with air, and on the nature of the ignition. Therefore, when assessing the preparedness of various combustible substances for a fire or explosion, in some cases it is advisable to focus on the limits of their concentration in the air, in others, in addition, on the flash or ignition temperature. With regard to explosion hazard, a distinction is made between heavy and light gases and vapors. The more dangerous are heavy gases and vapors with a density in relation to air of 1.5-2, having a lower explosive limit of about 2-3% and a low self-ignition temperature, and for flammable liquids vapors - also a low flash point. Light gases and vapors with a density of 0.8 or less in relation to air, having a lower explosive limit of 5-15% and a higher autoignition temperature, can be classified as less hazardous.
3. Flash - rapid combustion of fuel without the formation of compressed gases. Most combustible substances burn in the gas and vapor phase. Therefore, the ignition of the substance begins with a flash. The minimum temperature at which a flash appears is one at which vapors or gases are formed above the surface of a combustible substance that can flash in the air from an ignition source (for example, a spark), but the rate of their formation and the amount of heat released during a short flash are not yet sufficient for subsequent combustion. The lower temperature limit of ignition (flash point) is the lowest temperature of a liquid at which a mixture of saturated vapors with air is formed, which can ignite when an ignition source is brought to it. The upper temperature limit of ignition is the highest temperature of the liquid at which a mixture of saturated vapors with air is formed, which can still ignite. Above this temperature, the liquid forms saturated vapors, which, when mixed with air in a closed volume, cannot ignite. At a flash point of up to 45 ° C, liquids are called flammable (FL), and above 45 ° C - flammable (FL).
4. Combustion - the initiation of combustion under the influence of a source of ignition without a flame.
5. Ignition - Combustion with the appearance of a flame. Ignition temperature is the minimum temperature at which a substance ignites from an open source of fire and continues to burn after it has been removed. This is the process of the occurrence of combustion when a part of a combustible substance is heated by an ignition source, and the rest of the mass, for example, wood, remains cold. The readiness of a combustible mixture for ignition is determined by the limiting content of vapors, dust or gaseous products in it, and for some substances, also by the temperature of the mixture. Combustible dust can be in a room in a suspended state (aerosol) or settled on different surfaces(airgel). Aerosols have a higher ignition temperature than aerogels due to their lower concentration per unit volume, as a result of which the conditions for the development of combustion can occur at a higher temperature.
6. Spontaneous combustion (distinguish between thermal, chemical and microbiological) - combustion without contact of a combustible substance with an ignition source as a result of the formation of heat in the combustible substance itself as a result of some processes or heat supply from outside and an increase in its temperature. For example, a substance ignites only due to the release of heat from internal chemical or biological processes occurring in it (brown coal, peat, sawdust).
7. Self-ignition - spontaneous combustion with the release of flame. Autoignition temperature - the minimum temperature at which ignition occurs in air due to the heat of a chemical reaction without bringing up an open source of fire. Depending on the autoignition temperature, 5 groups of explosive mixtures are set. At a self-ignition temperature above 450 ° C, substances that form an explosive mixture with air belong to group T1; at a self-ignition temperature from 300 to 450 ° C - to T2; at temperatures from 200 to 300 ° C - to T3; over 135 to 200 ° C - to T4 and from 100 to 135 ° C - to T5. So, for example, methane gas is assigned to category 1 and to group T1, amyl acetate - to category 1 and group T2, turpentine - to category 1 and group T3. Acetone, carbon monoxide - category 2, group T1; gasoline B-72 - category 2, group T3; coke oven gas - category 3, group T1; hydrogen - category 4a, group T1; hydrogen sulfide - category 4a, group T3; acetylene - category 4b, group T2, etc. The distribution of explosive mixtures by categories and groups is given in full in the PIVRE. Self-ignition possible combustible dust... For example, coal dust in the form of an aerosol has a self-ignition temperature of 969 ° C, in the form of an airgel it ignites spontaneously at a temperature of 260 ° C. Spontaneous ignition of aerosols depends on the concentration of dust in the air and on the degree of particle size reduction. In the tables below. 1,2,3 are given the characteristics of the fire hazard of some gases, liquids and solids, which are often found in the operation of electrical installations. Solid combustible substances and liquids require not only the required concentration, but also a certain temperature for their ignition.
|
Table 1. Fire hazard characteristics of gases |
||||
|
Density in relation to air |
||||
|
Acetylene |
||||
|
Carbon monoxide |
||||
|
Hydrogen sulfide |
||||
|
Natural gas |
||||
|
Generator gas from lump fuels (coal, peat, wood) |
|
Table 2. Fire hazard characteristics of liquids |
|||||
|
Liquids |
Autoignition temperature, ° С |
Temperature limits of ignition (flash point), ° С |
Flammable concentration limits in percent by volume |
||
|
Gasoline A-74 |
|||||
|
Transformer oil |
|||||
|
Ethanol |
3. The concept of fire as a process
3.1. General information about burning
Combustion is a complex physicochemical process of interaction of a combustible substance and an oxidizer, characterized by a self-accelerating chemical transformation and accompanied by the release of a large amount of heat and light. Flame combustion can occur either under the influence of an ignition source (ignition), or as a result of a sharp increase in the rate of exothermic reactions (self-ignition).
Self-ignition mode consists in the spontaneous occurrence of flame combustion of a combustible mixture preheated to a certain critical temperature (the so-called autoignition temperature); this mode manifests itself in the form of a flash and is characterized by the simultaneous combustion of the entire combustible mixture. Table 1 lists some flammable substances and their autoignition temperatures.
Table 1.
Autoignition temperature of some combustible substances
Substance | Temperature, ° С | Substance | Temperature, ° С |
Wood | Aviation gasoline | ||
Sunflower oil | |||
Ethanol | |||
Ignition mode represents the propagation of a combustion wave (propagation of a flame front) through a cold mixture when it is locally ignited (ignited) by an external source. A flame is the visible combustion zone in which glow and heat emission are observed. The resulting flame itself becomes a source of heat flux and reactive particles into the adjacent layers of the fresh combustible mixture, thereby ensuring the movement of the flame front.
About spontaneous combustion of plant products... From plant products hay, straw, leaves, malt, hops are prone to spontaneous combustion. Particularly susceptible to spontaneous combustion are undried plant products, in which the vital activity of plant cells continues.
According to the bacterial theory, the presence of moisture and an increase in temperature due to the vital activity of plant cells promotes the multiplication of microorganisms present in plant products. Due to the poor thermal conductivity of plant products, the released heat gradually accumulates and the temperature in the mass of the product rises. At elevated temperatures, microorganisms die and turn into porous coal, which has the property of heating up due to intense oxidation and therefore is the next source of heat generation after microorganisms. The temperature in plant products rises to 300 ° C, and they ignite spontaneously.
Wood, lignite and bituminous coal, peat also ignite spontaneously due to intensive oxidation with atmospheric oxygen.
Vegetable and animal fats, if applied to crushed or fibrous materials (rags, ropes, tow, matting, wool, sawdust, soot, etc.) have the ability to ignite spontaneously.
When crushed or fibrous materials are wetted with oil, it spreads over the surface and, in contact with air, begins to oxidize. Simultaneously with oxidation in the oil, a polymerization process occurs (combining several molecules into one). Both the first and the second processes are accompanied by a significant release of heat. If the generated heat is not dissipated, i.e. builds up inside a tightly packed bale, the temperature in the oily material rises and can reach autoignition temperature.
Combustion occurs when there are three essential components: combustible substance, oxidizer and ignition source. Let's dwell on each of them in more detail.
Under the term combustible substance a substance is meant that is capable of self-burning after the external ignition source has been removed. The combustible substance can be in a solid, liquid or gaseous state. Most organic substances, a number of gaseous inorganic compounds and substances, many metals, etc. are combustible substances. Gases pose the greatest fire and explosion hazard.
Combustion of liquid. To ignite a flammable liquid above its surface, a vapor-air mixture must first form. Combustion of liquids is possible only in the vapor phase, while the surface of the liquid itself remains relatively cold. Among flammable liquids (FL), a class of the most dangerous representatives is distinguished - flammable liquids (FL). Flammable liquids include gasolines, acetone, benzene, toluene, some alcohols, ethers, etc.
There are a number of substances (gaseous, liquid or solid) that can spontaneously ignite on contact with air without preheating (at room temperature), such substances are called pyrophoric. These include: hydrogen fluoride, white phosphorus, hydrides and organometallic compounds of light metals, etc.
There is a fairly large group of substances, upon contact of which with water or water vapor in the air, a chemical reaction begins, proceeding with the release of a large amount of heat. Under the influence of the released heat, self-ignition of combustible reaction products and initial substances occurs. This group of substances includes alkali and alkaline earth metals (lithium, sodium, potassium, calcium, strontium, uranium, etc.), hydrides, carbides, phosphides of these metals, low molecular weight organometallic compounds (triethylaluminum, triisobutylaluminum, triethylboron), etc.
Combustion of solid occurs according to a more complex mechanism and several stages are inherent in it. When exposed to an external source, the surface layer of the solid is heated, and gaseous volatile products are released from it. This process can be accompanied either by the melting of the surface layer of the solid, or by its sublimation (the formation of gases, bypassing the melting stage). When a certain concentration of combustible gases in the air (lower concentration limit) is reached, they ignite and, by means of the released heat, begin to act on the surface layer themselves, causing it to melt and new portions of combustible gases and solid vapors entering the combustion zone.
Consider wood as an example. When heated to 110 ° C, the wood dries up and the resin evaporates slightly. Weak decomposition begins at 130 ° C. More noticeable wood decomposition (color change) occurs at 150 ° C and above. The decomposition products formed at 150-200 ° C are mainly water and carbon dioxide, therefore they cannot burn. At temperatures above 200 ° C, the main component of wood, fiber, begins to decompose. The gases produced at these temperatures are flammable as they contain significant amounts of carbon monoxide, hydrogen, hydrocarbons and other organic vapors. When the concentration of these products in the air becomes sufficient, under certain conditions they will ignite.
If a combustible substance melts while spreading, it increases the combustion site (for example, rubber, rubber, metals, etc.). In the event that the substance does not melt, oxygen gradually comes to the surface of the fuel and the process takes the form of heterogeneous combustion (the stage of burning out the coke of the carbon fuel). The combustion process of solids is complex and diverse, it depends on many factors (dispersion of a solid material, its moisture content, the presence of an oxide film on its surface and its strength, the presence of impurities, etc.).
More intensively (often with an explosion), fine-dispersed metal powders and dust-like combustible materials (for example, wood dust, powdered sugar) are ignited.
How oxidizing agent most often, during a fire, oxygen is released, the content of which in the air, as is known, is about 21%. Strong oxidizing agents are hydrogen peroxide, nitric and sulfuric acids, fluorine, bromine, chlorine and their gaseous compounds, chromic anhydride, potassium permanganate, chlorates and other compounds.
When interacting with metals, which exhibit very high activity in the molten state, water, carbon dioxide and other oxygen-containing compounds, which in common practice are considered inert, act as oxidants.
However, only the presence of a mixture of fuel and oxidizer is not enough to start the combustion process. Still needed ignition source... In order for a chemical reaction to occur, a sufficient number of active molecules, their fragments (radicals) or free atoms (which have not yet managed to combine into molecules), which have excess energy equal to or exceeding the activation energy for a given system, must appear.
The appearance of active atoms and molecules is possible when the entire system is heated, when gases are in local contact with a heated surface, when exposed to a flame, an electric discharge (spark or arc), local heating of the vessel wall as a result of friction or when a catalyst is introduced, etc. The source of ignition can also be the sudden adiabatic (without heat exchange with the environment) compression of the gas system or the impact of a shock wave on it.
Currently, scientists have established that the mechanism of the occurrence and development of real fires and explosions is characterized by a combined chain-thermal process. Having started in a chain way, the oxidation reaction, due to its exothermicity, continues to accelerate due to heat. Ultimately, the critical (limiting) conditions for the onset and development of combustion will be determined by the heat release and the conditions of heat and mass transfer of the reacting system with the environment.
3.2. Combustion cessation mechanism
The combustion cessation mechanism is understood as a system of factors leading to the end of the combustion process (reaction).
The mechanism for stopping combustion can be naturally conditioned when it is realized without human participation (self-liquidation of combustion, for example, in nature). At the same time, knowledge of the essence of the mechanism for stopping combustion allows you to purposefully use its factors both when eliminating small foci of combustion and when extinguishing fires.
To stop burning, at least one of the conditions must be met:
stop the flow of new portions of fuel vapors into the combustion zone;
stop the flow of oxidizer (oxygen in the air);
reduce the heat flux from the flame torch;
to reduce the concentration of active particles (radicals) in the combustion zone.
Based on this, one of the possible principles (methods) of extinguishing the fire can be:
lowering the temperature of the combustion source below the self-ignition temperature or the flash point of the fuel by introducing substances into the flame that, as a result of evaporation, sublimation or decomposition, take on a certain amount of heat (the classic substance is water);
reducing the amount of fuel vapors entering the combustion zone by isolating the combustible substance from the effect of the torch of the combustion seat (for example, using a dense blanket);
reducing the concentration of oxygen in the gas environment by diluting the medium with non-combustible additives (for example, nitrogen, carbon dioxide);
reducing the rate of the chemical reaction of oxidation due to the binding of active radicals and interrupting the chain reaction of combustion in the flame by introducing special chemically active substances (inhibitors);
creation of conditions for extinguishing a flame when it passes through narrow channels between particles extinguishing agent(fire barrier effect);
flame failure as a result of the dynamic effect of a jet of extinguishing agent on the fire.
As a rule, when a fire extinguishing agent acts on a fire source, no one mechanism of action is found in its pure form, the extinguishing process has a combined character. So the foam has an insulating and cooling effect, powder formulations have an inhibitory, fire-blocking and dynamic effect.
3.3 Classification of fires
All fires, depending on the state of aggregation of the combustible substances involved in the combustion process, are divided into several classes and denote them in capital Latin letters A, B, C, D, E. Characteristics of fire classes and pictograms used for them designations are given in the appendix.
Depending on the type of charged substance, fire extinguishers can be used to extinguish one or several classes of ignition:
class A ignition of solid combustible substances
Class B ignition of liquid combustible substances
class C ignition of gaseous combustible substances
Class D Combustion of metals and metal-containing substances
class E ignition of electrical installations under voltage.
It should be noted that the given classification almost coincides with that approved by the international standard ISO 3941. В international standard there are no subclasses, and there is no class "E", but there is a class "F", which denotes fires that can occur in food preparation areas of food objects. It should be borne in mind that the national classification in some countries differs from the international one. So in the USA the letter “A” denotes fires of solid combustible substances, the letter “B” fires of liquid and gaseous substances, but the letter “C” fires of electrical equipment under voltage, the letter “D” fires of metals and metal-containing substances. Therefore, when you pick up a fire extinguisher, be sure to look at its label, consider the pictograms of the classes of fires this fire extinguisher is designed to extinguish.
The pictograms of the classes of fires for which the extinguisher cannot be used to extinguish, are either crossed out with a diagonal stripe, or not shown at all.
3.4. Dangerous factors of fire
In accordance with GOST 12.01.004-85 "Fire safety" hazardous factors of fire are: flames and sparks, high ambient temperature, toxic products of combustion and thermal decomposition, smoke, low oxygen concentration.
Flame
Combustion of all liquid, gaseous and most solid combustible substances, which, decomposing or evaporating, emit gaseous products, is accompanied by the formation of a flame. Thus, the flame is a gas volume in which the process of combustion of vapors and gases takes place.
Solids burn without flame: graphite, anthracite, coke, soot, charcoal. These substances do not decompose and do not form gases when heated, or form them in quantities insufficient for combustion.
The glow of a flame when burning organic substances depends on the presence of incandescent solid particles of carbon in it, which have time to burn. A non-luminous (blue) flame usually occurs during the combustion of gaseous products: carbon monoxide, hydrogen, methane, ammonia, hydrogen sulfide.
The flame temperature during combustion of some combustible substances in air is: wood - 850-1400 ° C, oil products in the tank - 1100-1300 ° C, carbon disulfide - 2195 ° C, stearin - 640-940 ° C.
Open fire is very dangerous for humans, because exposure of the body to flame causes burns. An even greater danger is the thermal radiation of a fire, which can cause burns to the body, eyes, etc.
Temperature
Inhalation of heated air leads to damage and necrosis of the upper respiratory tract, suffocation and death of a person. When exposed to temperatures above 100 ° C, a person loses consciousness and dies in a few minutes.
Skin burns are dangerous for humans. Despite the great success of medicine in their treatment, a victim who has received second-degree burns on 30% of the body surface has little chance of surviving. The time for which a person receives second-degree burns is short: at an ambient temperature of 71 ° C - 26 seconds, at 100 ° C - 15 seconds. Studies have found that in a humid atmosphere typical of a fire, a second degree burn is caused by a temperature significantly lower than indicated. Thus, an ambient temperature of 60-70 ° C is dangerous to human life, and not only in a burning room, but also in adjacent rooms, into which combustion products and heated air have entered.
Decreased oxygen concentration
Most often, people die in fires not from fire and high temperatures, but due to a decrease in the concentration of oxygen in the air and poisoning with toxic combustion products.
The first symptoms of oxygen deficiency (increased breathing volume, decreased attention, impaired muscle coordination) are observed in people with an oxygen content in the inhaled gas mixture at the level of 16-17%. A decrease in the concentration of O 2 to 12-15% causes shortness of breath, increased heart rate, deterioration of mental activity, dizziness, and rapid fatigability. In cases when the concentration of O 2 decreases to 10-12%, consciousness remains, but nausea, severe fatigue appear, breathing becomes intermittent. At a concentration of 8%, loss of consciousness occurs quickly, and below 6%, death occurs within 6-8 minutes.
Toxic combustion products
This topic will be more fully disclosed by specialists (Chemist, Toxicologist).
How dangerous toxic combustion products are, is clearly shown by the example of a fire that occurred in a clothing store in Tokyo (Japan). A fire broke out on the 3rd floor, and 118 people died in a bar located on the 7th floor of the same building, 96 of them from poisoning with toxic combustion products, 22 people jumped out of windows. Many people fainted within the first 2-3 minutes; their death occurred in 4-5 minutes. after losing consciousness.
Smoke
Smoke is dangerous not only for the toxic substances it contains, but also for reduced visibility. This makes it difficult, and sometimes makes it almost impossible to evacuate people from a dangerous room. People need to see clearly to get to safety quickly. emergency exits or their pointers.
If visibility is lost, organized movement (especially in an unfamiliar building, on objects with mass stay people) is violated, becomes chaotic, everyone moves in an arbitrarily chosen direction. Panic ensues. People are seized by fear, suppressing consciousness, will. In this state, a person loses the ability to navigate, correctly assess the situation.
Explosion
One of the types of instant combustion is the explosion of special explosives, as well as a mixture of flammable gases, vapors or dust with air. These are chemical explosions.
Physical explosions are ruptures various containers and apparatuses (boilers, tanks, cylinders, etc.) resulting from the development of excessive pressure by gases or vapors exceeding the pressure that the walls of tanks and apparatuses can withstand.
At the moment of an explosion of a chemical nature, the substance burns out at a high speed, and the resulting gases and vapors expand strongly and create great pressure on the environment. This explains the tremendous force of destruction caused by the explosion. An explosion usually creates a flame, which can ignite nearby combustible substances.
1.3 Conditions of fire, dangerous factors fire
Fire conditions
Fires occur where a person uses fire for his daily needs and where, due to violations of fire safety rules, the fire gets out of his control.
Most often, fires occur due to the so-called human factor. This happens when people, due to their illiteracy in the field fire safety, negligence and indiscipline violate fire safety rules in everyday life.
The human factor turns fire into a terrible, all-destructive element.
Fire becomes the enemy of man:
■ if the use of it in the process of life is treated irresponsibly;
■ if the established fire safety standards are not observed;
■ if they try to use the power of fire not for creation, but for destruction (arson, armed conflicts),
■ if control over the combustion process is lost.
As soon as the fire breaks out of human control, a fire breaks out with all the ensuing consequences.
Dangerous factors of fire
· Open fire ignites flammable materials, clothing;
· The high temperature of the heated air causes burns of the respiratory tract, human body;
· Carbon monoxide causes loss of consciousness and death of a person;
· Poisonous substances formed from the combustion of synthetic materials lead to poisoning of the human body and its death;
· Smoke creates poor visibility conditions for finding escape routes;
· The collapse of building structures leads to the death of a person.
As a result of exposure damaging factors fire occurs combustion of objects and objects, their charring, destruction, failure. Elements of buildings and structures made of combustible materials are destroyed. Action high temperatures causes burnout, deformation and collapse metal trusses, floor beams, other structural details of structures. In case of fires, technological equipment is completely or partially destroyed and vehicles... People die or receive burns of varying severity.
Secondary consequences of fires can be explosions, release of toxic or pollutants into the environment. Water used to extinguish the fire can cause great damage to premises not affected by the fire. A serious social and economic consequence of the fire is the termination of the object of performing its economic and other functions.
Combustion process and types of combustion
By burning is called a fast chemical process of oxidation or combination of a combustible substance and oxygen in the air, accompanied by the release of gas, heat and light.
Combustion is known even without oxygen in air with the formation of heat and light. Thus, combustion is not only chemical reaction compounds, but also decomposition.
Distinguish between actual combustion, explosion and detonation. During combustion itself, the flame propagation speed does not exceed tens of meters per second, during an explosion - hundreds of meters per second, and during detonation - thousands of meters per second.
Combustion is fastest in pure oxygen. As the concentration of oxygen decreases, the combustion process slows down, the lowest combustion rate when the oxygen content in the air is 14–15%.
Burning requires combustible materials, oxidizer and ignition source.
In practice, complete and incomplete combustion are distinguished. Complete combustion is achieved with a sufficient amount of oxygen, and incomplete combustion with a lack of oxygen. Incomplete combustion, as a rule, forms caustic, toxic and explosive mixtures.
· Take all feasible measures to save people, property and extinguish fires before the arrival of the fire brigade;
· Render assistance to the fire department in extinguishing a fire;
· Comply with the legal requirements of fire officials.
FIRE IMMEDIATELY REPORT THE FIRE DEPARTMENT BY PHONE 01.
Fire safety requirements in nature
Forest fires can be caused by lightning or careless human activities. Such fires are very dangerous, and in dry, hot weather, they can become natural disasters.
There are two types of fires: forest fires (downstream or upstream) and peat fires. Grassroots forest fires usually occur in deciduous forests; the speed of fire propagation is low, and the height of the flame can reach 2 m. Horse bush fire is typical for coniferous forests. The rate of spread of fire is higher than in the forest grassland fire, and in windy weather it can be very high (25-30 km / h). Peat fires occur on drained or natural peat bogs. They are characterized by prolonged smoldering of peat and the occurrence of strong air smoke. Peat is a highly flammable material, therefore such fires are very dangerous.
According to statistics, most forest fires occur due to human negligence. In order to prevent their occurrence, it is necessary to follow several important rules.
In a fire hazardous period, do not use open fire in the forest!
These periods include mid and late spring (forest soil is covered with dry leaves and grass), as well as all summer and early autumn, when the weather is hot and there is no rain for more than a week. Do not make a fire in places where there is a lot of dry grass, in young coniferous stands, in forest areas not cleared of felling residues.
If a special fire-fighting mode, it is strictly forbidden to visit the forests until it is canceled.
Do not bring flammable liquids or materials soaked with them into the forest. Do not leave any glass shards in the forest: if hit sun rays these debris can focus them, causing a fire.
1.5 Procedure in the event of a fire
Citizens are obliged:
Comply with fire safety requirements;
Have in the premises and buildings that they own primary funds fire extinguishing;
If fires are detected, notify them immediately fire brigade;
Before the arrival of the fire brigade, take a feasible part in extinguishing a fire, saving people and property;
Provide assistance to the fire brigade in extinguishing a fire;
Comply with the orders, regulations and other legal requirements of officials of the state fire supervision.
Fire brigade call rules:
Immediately inform the fire department about the occurrence of a fire by calling "01". When calling for help, you must:
• briefly and clearly outline the event - what is on fire;
· Name the address, street, house, apartment;
· Give your surname, phone number;
· If you do not have access to the telephone and there is no way to leave the room, open the window and shouting to attract the attention of passers-by.
Actions in case of fire:
· Report the fire by phone "01";
· Evacuate people, report the fire to neighbors;
· If possible, take measures to extinguish the fire (de-energize the premises, use primary fire extinguishing means).
· In a fire, people die mainly not from the effects of open fire, but from smoke, so protect yourself from it by all means;
· Bend down to the floor - there remains a layer of air of 15-20 cm;
Breathe through a wet cloth or towel;
· In smoke, it is best to crawl along the wall towards the exit from the building.
· Leave children unattended from the moment a fire is detected until it is extinguished.
· Fight the flames yourself without calling the fire department.
· Use the elevators.
The worst thing in a fire is confusion and panic. Precious minutes pass away when fire and smoke leave less and less chance of getting out to safety. This is why everyone should know what to do when a fire breaks out.
The fire happened in the apartment
· Immediately call the firefighters by calling 01, tell them your exact address (street, house and apartment number, floor, entrance, code) and what is on.
· If there is no telephone, report the fire through your neighbors.
Remember: the most important thing in this situation is not to lose your composure and not to panic!
· Without waiting for the arrival of firefighters, try to extinguish the fire with available means (water, thick wet cloth, water from internal fire hydrants on staircases).
· On staircase open the fire hydrant locker (labeled PC). Grasping the barrel of the sleeve, roll it towards the fire. Turn the valve of the fire cock counterclockwise until it stops and let the water into the hose, go to the trunk and start extinguishing the fire. Direct the water jet to the places of the strongest burning. Change the direction of the water jet from time to time to prevent the spread of fire (do not pour water over smoke or over the top of the flame).
Extinguish flammable liquids with a wet cloth, sand, earth from flower pots, washing powder.
· Do not open windows and doors in order not to increase the flow of air to the fire site.
Do not extinguish electrical appliances connected to the mains with water and pour water on electric wires, to avoid electric shock, turn off the power.
· If it is not possible to eliminate the combustion center on your own, then you must immediately leave the apartment, closing the door behind you.
· When leaving the apartment, it is necessary to move on all fours along the smoky corridor (there is less smoke below) and breathe through a damp cloth; to protect yourself from fire, you should cover yourself with a damp cloth (wet blanket, coat)
· After leaving the apartment, arrange a meeting of the firefighters, point them to the source of the fire.
· If it is impossible to leave the apartment in the usual way, use the balcony fire escape. If there is no balcony fire escape, you need to go to the balcony, close the door tightly and call for help.
When leaving the building in the event of a fire, do not use
· By an elevator, it may switch off.
TV set on fire
· If you smell smoke, turn off the power to the TV.
· If access to the outlet is not possible, switch off the power switch in the electrical panel.
· Inform the fire brigade about the fire by phone 01, indicate the exact address (street, house and apartment number, floor, entrance, code) and what is on.
· If, after disconnecting from the power, the combustion does not stop, then fill the TV with water through the hole in the rear wall. While doing this, stand to the side of the TV.
· To avoid poisoning when extinguishing a lit TV, breathe through a damp towel (cloth).
· After extinguishing the fire, ventilate the area before the firefighters arrive.
· Inform your parents about the fire, do not touch anything until the arrival of the firefighters, who will establish the cause of the fire and give their opinion.
Fire on the balcony
· Immediately call the fire brigade at phone number 01 or the unified mobile phone number 112, indicate your exact address and what is lit.
· Extinguish the fire with any available means (wet cloth, water).
· Extinguish flammable liquids with a wet cloth, sand, earth from flower pots, washing powder.
· Warn the neighbors of the upper floors, call them for help.
The smell of smoke in the entrance
· Immediately call the fire department on 01, giving your exact address and what is lit.
· Try to locate the burning site (mailboxes, garbage chute, elevator, apartment) and inform your neighbors about the fire.
· In a smoky room, you can move either on all fours or crawling; to protect the respiratory system, you must breathe through a wet cloth.
· Together with your neighbors, try to localize the fire and extinguish it with improvised means.
· If a fire occurred outside your apartment and it is impossible to use the stairs to go outside, stay in the apartment.
· Cover door openings and ventilation openings with wet blankets, towels, etc. to avoid poisoning by combustion products.
You can also hide from the fire before the arrival of firefighters on the balcony, tightly closing it behind you balcony door... Before that, you must close all doors in your apartment to prevent the spread of smoke.
· When firefighters arrive, call their attention and ask for help.
Fire in the elevator car
· In case of fire in the elevator car or shaft, it is necessary to immediately inform the dispatcher about it by pressing the "Call" button.
· If the elevator is moving, do not stop it, but wait until it stops.
· After leaving the elevator car, lock the doors and ask the residents on the floor to call the fire department.
When extinguishing a fire, do not enter the cockpit; to extinguish the fire, use a dense dry cloth, dry sand, carbon dioxide (OU-2, OU-5) or powder fire extinguisher(OP-1, OP-2 "Moment").
· If the elevator stops between floors, and the fire source is outside the car, knock on the walls of the car, shout and call for help, try with the help of the residents to open the automatic elevator doors and get out.
· If it is impossible to get out of the elevator on your own before the arrival of help, close your nose and mouth with a handkerchief, a sleeve of clothing, moistened with liquid (even urine), maintain restraint and calmness.
Broke out Christmas tree
· If an electric garland lights up on a tree, immediately disconnect the garland from the mains by pulling out the power plug from the socket.
· Call the fire brigade by phone 01, specifying the exact address and what is lit.
· Dump the burning Christmas tree on the floor, throw a simple blanket over it (no filler) and fill it with water.
· Use water and sand to extinguish the fire or prevent the fire from spreading before firefighters arrive.
· If it is impossible to extinguish the fire, then leave the burning room, close the door tightly behind you and pour water on the door outside.
· Report the fire to your neighbors, if necessary, leave the apartment and wait for the firefighters.
rules safe behavior in case of fire in public places
Being in any public place, try to remember the route to the exit.
Pay attention to the plan for evacuating people in the event of a fire, try to
Provide direction and number of paths possible evacuation, location of staircases and emergency exits.
Pay attention to the presence in the corridors and staircases of lamps painted in green color... These are emergency lighting lamps for evacuation during a fire.
· Hearing the cries of "Fire!", Keep calm and self-control.
· Look around, assessing the situation. Noticing a phone or a button fire alarm, report the fire to the fire department.
If the room is full of smoke or if there is no lighting, move forward
· To the exit, holding onto the walls, handrails. Breathe through a handkerchief or sleeve. If you are in a multi-story building, do not try to use the elevators, go down the stairs.
Do not jump out of a window with great height(above the second floor). If it is impossible to go outside, retreat into a room where there is no fire, close the door tightly and wait for the help of the firefighters there.
· When clothes catch fire, do not run under any circumstances (this will intensify the flame). Take off burning clothing. Help the one on whom personal items and clothing caught fire.
If you cannot throw off the burning clothes, put a
thick fabric (coat, bedspread, etc.), leaving him
wu open so that he does not suffocate from the combustion products. When
lack of dense fabric, you just need to roll on the floor.
What to do in case of a fire in the forest
· If you find a fire in the forest, immediately report it to the rescue service, the rural district administration or the forestry. Remember the two numbers to call in case forest fire: 01 and 112 (for mobile phones only).
· If the fire you discovered has not yet gained strength, take measures to extinguish it with water, earth, sand, branches of deciduous trees, thick clothing. Most effective method extinguishing a forest fire - throwing earth at the edge of the fire.
· When extinguishing a forest fire, do not go far from roads and clearings, keep in touch with other participants in extinguishing the fire using visual and sound signals.
· If the fire has flared up too much and you are unable to stop it, immediately leave the scene.
· In case of a forest ground fire, you need to move perpendicular to the direction of the fire, along clearings, roads, river banks or glades.
· In a forest riding fire, move through the forest with a crouch to the ground and cover your airways with a damp cloth.
· If you have no way to get out of the danger zone, try to find some body of water in the forest and enter it.
· Sometimes a fire can turn into a real natural disaster, which even special services are not immediately able to cope with. If a fire begins to approach a populated area, collective measures must be taken to extinguish it. The most extreme measure is the immediate evacuation of the inhabitants of this settlement... In this case, you must obey the emergency workers without question. Don't panic and wait for help. If it is impossible to take personal property with you, bury it in the ground. It is best to wait for help on large open spaces or in special shelters.
· If your clothes are on fire, do not run!
· From this, the flame flares up much faster. Try to remove burning clothing. If you fail to do this, lie on the ground and roll to put out the fire.
· If you see that clothing caught fire on another person, do not let him run and try to remove the burning clothing from him. If it is not possible to remove clothing from him, knock the victim to the ground and extinguish the flame with any possible way: cover thick cloth, cover with water, cover with sand or earth.
Security measures
1. Keep your yard clean. Do not store combustible material near houses or attics. Have the first extinguishing media (water, sand). Do not use matches, candles and lamps to illuminate various kinds of premises.
2. Avoid lighting stoves, kerosene stoves, kerosene stoves in rooms, especially near furniture, curtains, wooden structures... Refuel them only with kerosene.
3. Avoid smoking in barns, garages, hayloft, attics and storerooms.
4. Do not leave heating stoves, lighting and electric heating devices.
5. Do not use electric stoves, fireplaces and other homemade heating devices as heating.
6. Clean the chimneys at least 2 times during the heating season.
7. Do not use firewood for heating, the length of which exceeds the dimensions of the firebox. Do not heat with open doors.
8. Do not overheat ovens! Do not dry wood, clothing, or other combustible materials on or near stoves.
9. Make coating and whitewashing chimneys in attics,
10. Do not pour burning ash, coals near buildings,
11. Do not entrust the heating of stoves to children who
12. In front of the furnace hole, nail a 50 x 70 cm sheet of iron to the floor.
14. Use only automatic and calibrated inserts to protect power grids, not bugs.
15. Have special fireproof stands for installing electric irons, electric stoves, electric kettles, etc.
16. Replace the old, unusable wiring with a new one.
17. Do not leave children unattended by adults.
18. Do not send children for matches and cigarettes.
19. Explain to the children what a childish prank with fire leads to.
20. Do not use celluloid or other flammable toys and ornaments to decorate the Christmas tree.
21. Do not bring flammable liquids or materials soaked with them into the forest.
22. Do not leave any glass shards in the forest.
23. Do not make fires during the fire season.
24. Do not make a fire near trees and bushes, dry dead wood, in windy weather.
25. Do not leave the fire untouched.
26. IMMEDIATELY REPORT THE FIRE TO THE FIRE DEPARTMENT BY PHONE 01.






